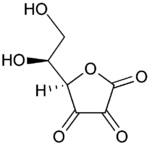
Vitamin C
| |
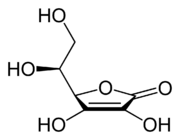
| |
Vitamin C
| |
| Systematic name | |
| IUPAC name 2-oxo-L-threo-hexono-1,4- lactone-2,3-enediol or (R)-3,4-dihydroxy-5-((S)- 1,2-dihydroxyethyl)furan-2(5H)-one | |
| Identifiers | |
| CAS number | 50-81-7 |
| ATC code | A11G |
| PubChem | 644104 |
| Chemical data | |
| Formula | C6H8O6 |
| Mol. weight | 176.13 grams per mol |
| Synonyms | L-ascorbate |
| Physical data | |
| Melt. point | 190°C (374°F) |
| Pharmacokinetic data | |
| Bioavailability | rapid & complete |
| Protein binding | negligible |
| Metabolism | ? |
| Half life | 30 minutes |
| Excretion | renal |
| Therapeutic considerations | |
| Pregnancy cat. | A |
| Legal status | general public availability |
| Routes | oral |
Vitamin C or L-ascorbate is an essential nutrient for higher primates, and a small number of other species. The presence of ascorbate is required for a range of essential metabolic reactions in all animals and in plants and is made internally by almost all organisms, (humans being one notable exception). It is widely known as the vitamin that prevents scurvy in humans.[1][2][3]
The pharmacophore of vitamin C is the ascorbate ion. In living organisms, ascorbate is an antioxidant, as it protects the body against oxidative stress,[4] and is a cofactor in several vital enzymatic reactions.[5]
As a nutrient, its uses and the daily requirement are matters of on-going debate. As a food additive, vitamin C is used as an antioxidant preservative and an acidity regulator. Several E numbers account for the vitamin, depending on its chemical structure: E300 as ascorbic acid, E301 as the salt sodium ascorbate, E302 as the salt calcium ascorbate, E303 as the salt potassium ascorbate, E304 for the esters ascorbyl palmitate and ascorbyl stearate, and E315 for the stereoisomer erythorbic acid.
Ascorbic acid
Ascorbic acid is an organic acid with antioxidant properties. Its appearance is white to light yellow crystals or powder. It is water soluble. The L-enantiomer of ascorbic acid is commonly known as vitamin C. The name is derived from a- and scorbuticus (Scurvy) as a shortage of this molecule may lead to scurvy. In 1937 the Nobel Prize for chemistry was awarded to Walter Haworth for his work in determining the structure of ascorbic acid (shared with Paul Karrer, who received his award for work on vitamins), and the prize for Physiology or Medicine that year went to Albert Szent-Györgyi for his studies of the biological functions of L-ascorbic acid. At the time of its discovery in the 1920s it was called hexuronic acid by some researchers.[6]
Ascorbic acid behaves as a vinylogous carboxylic acid, where the double bond ("vinyl") transmits electron pairs between the hydroxyl and the carbonyl. There are two resonance structures for the deprotonated form, differing in the position of the double bond.
Another way to look at ascorbic acid is to consider it as an enol. The deprotonated form is an enolate, which are usually strongly basic. However, the adjacent double bond stabilizes the deprotonated form.
Ascorbic acid also rapidly interconverts into two unstable diketone tautomers by proton transfer, although it is the most stable in the enol form. The proton of the enol is lost, and reacquired by electrons from the double bond, to produce a diketone. This is an enol reaction. There are two possible forms, 1,2-diketone and 1,3-diketone.
The concentration of a solution of ascorbic acid can be determined in many ways, the most common ways involve titration with an oxidizing agent.
- DCPIP
A commonly used oxidising agent is the dye 2,6-dichlorophenol-indophenol, or DCPIP for short. The blue dye is run into the ascorbic acid solution until a faint pink colour persists for 15 seconds.
- Iodine
Another method involves using iodine and a starch indicator where iodine reacts with ascorbic acid and when all the ascorbic acid has reacted the iodine is then in excess, forming a blue-black complex with the starch indicator. This indicates the end point of the titration. Alternatively, ascorbic acid can be reacted with iodine in excess, followed by back titration with sodium thiosulfate while using starch as an indicator.
- Iodate and iodine
The above method involving iodine requires making up and standardising the iodine solution. One way round this is to generate the iodine in the presence of the ascorbic acid by the reaction of iodate and iodide ion in acid solution.
- N-Bromosuccinimide
A much less common oxidising agent is N-bromosuccinimide, (NBS). In this titration the NBS oxidises the ascorbic acid (in the presence of potassium iodide and starch). When the NBS is in excess (i.e. the reaction is complete) the NBS liberates the iodine from the potassium iodide which then forms the blue/black complex with starch, indicating the end point of the titration.
Biological significance
Vitamin C is purely the L-enantiomer of ascorbate; the opposite D-enantiomer has no physiological significance. Both forms are mirror images of the same molecular structure. When L-ascorbate, which is a strong reducing agent carries out its reducing function, it is converted to its oxidized form, L-dehydroascorbate.[5] L-dehydroscorbate can then be reduced back to the active L-ascorbate form in the body by enzymes and glutathione.[7]
L-ascorbate is a weak sugar acid structurally related to glucose which naturally occurs either attached to a hydrogen ion, forming ascorbic acid, or to a metal ion, forming a mineral ascorbate.
Function
In humans, vitamin C is a highly effective antioxidant, acting to lessen oxidative stress, a substrate for ascorbate peroxidase,[3] as well as an enzyme cofactor for the biosynthesis of many important biochemicals. Vitamin C acts as an electron donor for eight different enzymes:[8]
- Three participate in collagen hydroxylation.[9][10][11] These reactions add hydroxyl groups to the amino acids proline or lysine in the collagen molecule (via prolyl hydroxylase and lysyl hydroxylase), thereby allowing the collagen molecule to assume its triple helix structure and making vitamin C essential to the development and maintenance of scar tissue, blood vessels, and cartilage.[12]
- Two are necessary for synthesis of carnitine.[13][14] Carnitine is essential for the transport of fatty acids into mitochondria for ATP generation.
- The remaining three have the following functions:
Biological tissues that accumulate over 100 times the level in blood plasma of vitamin C are the adrenal glands, pituitary, thymus, corpus luteum, and retina.[21] Those with 10 to 50 times the concentration present in blood plasma include the brain, spleen, lung, testicle, lymph nodes, liver, thyroid, small intestinal mucosa, leukocytes, pancreas, kidney and salivary glands.
Biosynthesis
The vast majority of animals and plants are able to synthesize their own vitamin C, through a sequence of four enzyme-driven steps, which convert glucose to vitamin C.[5] The glucose needed to produce ascorbate in the liver (in mammals and perching birds) is extracted from glycogen; ascorbate synthesis is a glycogenolysis-dependent process.[22] In reptiles and birds the biosynthesis is carried out in the kidneys.
Among the animals that have lost the ability to synthesise vitamin C are simians, guinea pigs, the red-vented bulbul,and fruit-eating bats.[5] Most notably, along with the rest of the ape family in which we reside, humans have no capability to manufacture vitamin C. The cause of this phenomenon is that the last enzyme in the synthesis process, L-gulonolactone oxidase, cannot be made by the listed animals because the gene for this enzyme, Pseudogene ΨGULO, is defective.[23] The mutation has not been lethal because vitamin C is prevalent in their food sources, with many of these species' natural diets consisting largely of fruit.
Most simians consume the vitamin in amounts 10 to 20 times higher than that recommended by governments for humans.[24] It has been noted that the loss of the ability to synthesize ascorbate strikingly parallels the evolutionary loss of the ability to break down uric acid. Uric acid and ascorbate are both strong reducing agents. This has led to the suggestion that in higher primates, uric acid has taken over some of the functions of ascorbate.[25] Ascorbic acid can be oxidised (broken down) in the human body by the enzyme ascorbic acid oxidase.
An adult goat, a typical example of a vitamin C-producing animal, will manufacture more than 13,000 mg of vitamin C per day in normal health and as much as 100,000 mg daily when faced with life-threatening disease, trauma or stress.[26] It is thought that the human Vitamin C requirement is far lower than that of Vitamin C-synthesizing mammals due to increased Vitamin C recycling efficiency. [27]
Trauma or injury has also been demonstrated to use up large quantities of vitamin C in humans.[28]
Some microorganisms such as the yeast Saccharomyces cerevisiae have been shown to be able to synthesize vitamin C from simple sugars.[29][30]
Deficiency
Scurvy is an avitaminosis resulting from lack of vitamin C, as without this vitamin, the synthesised collagen is too unstable to meet its function. Scurvy leads to the formation of liver spots on the skin, spongy gums, and bleeding from all mucous membranes. The spots are most abundant on the thighs and legs, and a person with the ailment looks pale, feels depressed, and is partially immobilized. In advanced scurvy there are open, suppurating wounds and loss of teeth and, eventually, death. The human body cannot store vitamin C,[31] and so the body soon depletes itself if fresh supplies are not consumed through the digestive system.
History of human understanding

The need to include fresh plant food or raw animal flesh in the diet to prevent disease was known from ancient times. Native peoples living in marginal areas incorporated this into their medicinal lore. For example, spruce needles were used in temperate zones in infusions, or the leaves from species of drought-resistant trees in desert areas. In 1536, the French explorer Jacques Cartier, exploring the St. Lawrence River, used the local natives' knowledge to save his men who were dying of scurvy. He boiled the needles of the arbor vitae tree to make a tea that was later shown to contain 50 mg of vitamin C per 100 grams.[32][33]
Throughout history, the benefit of plant food to survive long sea voyages has been occasionally recommended by authorities. John Woodall, the first appointed surgeon to the British East India Company, recommended the preventive and curative use of lemon juice in his book "The Surgeon's Mate", in 1617. The Dutch writer, Johann Bachstrom, in 1734, gave the firm opinion that "scurvy is solely owing to a total abstinence from fresh vegetable food, and greens; which is alone the primary cause of the disease."
While the earliest documented case of scurvy was described by Hippocrates around the year 400 B.C.E., the first attempt to give scientific basis for the cause of this disease was by a ship's surgeon in the British Royal Navy, James Lind. Scurvy was common among those with poor access to fresh fruit and vegetables, such as remote, isolated sailors and soldiers. While at sea in May 1747, Lind provided some crew members with two oranges and one lemon per day, in addition to normal rations, while others continued on cider, vinegar, sulfuric acid or seawater, along with their normal rations. In the history of science this is considered to be the first occurrence of a controlled experiment comparing results on two populations of a factor applied to one group only with all other factors the same. The results conclusively showed that citrus fruits prevented the disease. Lind published his work in 1753 in his Treatise on the Scurvy.

Lind's work was slow to be noticed, partly because he gave conflicting evidence within the book, and partly because the British admiralty saw care for the well-being of crews as a sign of weakness. In addition, fresh fruit was very expensive to keep on board, whereas boiling it down to juice allowed easy storage but destroyed the vitamin (especially if boiled in copper kettles[34]). Ship captains assumed wrongly that Lind's suggestions didn't work because those juices failed to cure scurvy.
It was 1795 before the British navy adopted lemons or lime as standard issue at sea. Limes were more popular as they could be found in British West Indian Colonies, unlike lemons which weren't found in British Dominions, and were therefore more expensive. This practice led to the use of the nickname "limey" to refer to the British. Captain James Cook had previously demonstrated and proven the principle of the advantages of fresh and preserved foods, such as sauerkraut, by taking his crews to the Hawaiian Islands and beyond without losing any of his men to scurvy. For this otherwise unheard of feat, the British Admiralty awarded him a medal.
The name "antiscorbutic" was used in the eighteenth and nineteenth centuries as general term for those foods known to prevent scurvy, even though there was no understanding of the reason for this. These foods included but were not limited to: lemons, limes, and oranges; sauerkraut, cabbage, malt, and portable soup.
In 1907, Axel Holst and Theodor Frølich, two Norwegian physicians studying beriberi contracted aboard ship's crews in the Norwegian Fishing Fleet, wanted a small test mammal to substitute for the pigeons they used. They fed guinea pigs their test diet, which had earlier produced beriberi in their pigeons, and were surprised when scurvy resulted instead. Until that time scurvy had not been observed in any organism apart from humans, and had been considered an exclusively human disease.
Discovery of ascorbic acid

In 1912, the Polish-American biochemist Casimir Funk, while researching deficiency diseases, developed the concept of vitamins to refer to the nutrients which are essential to health. Then, from 1928 to 1933, the Hungarian research team of Joseph L Svirbely and Albert Szent-Györgyi and, independently, the American Charles Glen King, first isolated vitamin C and showed it to be ascorbic acid. For this, Szent-Györgyi was awarded the 1937 Nobel Prize in Medicine.[35]
In 1928 the Arctic anthropologist Vilhjalmur Stefansson attempted to prove his theory of how the Eskimos are able to avoid scurvy with almost no plant food in their diet, despite the disease striking European Arctic explorers living on similar high-meat diets. Stefansson theorised that the natives get their vitamin C from fresh meat that is minimally cooked. Starting in February 1928, for one year he and a colleague lived on an exclusively minimally-cooked meat diet while under medical supervision; they remained healthy.
Between 1933 and 1934, the British chemists Sir Walter Norman Haworth and Sir Edmund Hirst and, independently, the Polish chemist Tadeus Reichstein, succeeded in synthesizing the vitamin, the first to be artificially produced. This made possible the cheap mass-production of vitamin C. Only Haworth was awarded the 1937 Nobel Prize in Chemistry for this work, but the process for vitamin C retained Reichstein's name.
In 1934 Hoffmann–La Roche became the first pharmaceutical company to mass-produce synthetic vitamin C, under the brand name of Redoxon.
In 1959 the American J.J. Burns showed that the reason some mammals were susceptible to scurvy was the inability of their liver to produce the active enzyme L-gulonolactone oxidase, which is the last of the chain of four enzymes which synthesize vitamin C.[36][37] American biochemist Irwin Stone was the first to exploit vitamin C for its food preservative properties. He later developed the theory that humans possess a mutated form of the L-gulonolactone oxidase coding gene.
Daily requirements
The North American Dietary Reference Intake recommends 90 milligrams per day and no more than 2 grams per day (2000 milligrams per day).[38] Other related species sharing the same inability to produce vitamin C and requiring exogenous vitamin C consume 20 to 80 times this reference intake.[39][40] There is continuing debate within the scientific community over the best dose schedule (the amount and frequency of intake) of vitamin C for maintaining optimal health in humans.[41] It is generally agreed that a balanced diet without supplementation contains enough vitamin C to prevent acute scurvy in an average healthy adult, while those who are pregnant, smoke tobacco, or are under stress require slightly more.[38]
High doses (thousands of milligrams) may result in diarrhea, which is harmless if the dose is reduced immediately. Some researchers[42] claim the onset of diarrhea to be an indication of where the body’s true vitamin C requirement lies. Both Cathcart[42] and Cameron have demonstrated that very sick patients with cancer or influenza do not display any evidence of diarrhea at all until ascorbate intake reaches levels as high as 200 grams (nearly half a pound).
| United States vitamin C recommendations[38] | |
|---|---|
| Recommended Dietary Allowance (adult male) | 90 mg per day |
| Recommended Dietary Allowance (adult female) | 75 mg per day |
| Tolerable Upper Intake Level (adult male) | 2000 mg per day |
| Tolerable Upper Intake Level (adult female) | 2000 mg per day |
Government recommended intakes
Recommendations for vitamin C intake have been set by various national agencies:
- 40 milligrams per day — the United Kingdom's Food Standards Agency[1]
- 45 milligrams per day — the World Health Organization[43]
- 60-95 milligrams per day — United States' National Academy of Sciences[38]
The United States defined Tolerable Upper Intake Level for a 25-year old male is 2000 milligrams per day.
Independent recommended intakes
Some independent researchers have calculated the amount needed for an adult human to achieve similar blood serum levels as vitamin C synthesising mammals as follows:
- 400 milligrams per day — the Linus Pauling Institute[44]
- 500 milligrams per 12 hours — Professor Roc Ordman, from research into biological free radicals[45]
- 3,000 milligrams per day (or up to 300,000 mg during illness or pregnancy) — the Vitamin C Foundation[46]
- 6,000–12,000 milligrams per day — Thomas E. Levy, Colorado Integrative Medical Centre.[47]
- 6,000–18,000 milligrams per day — Linus Pauling's personal use[48]
- 3,000–200,000 milligrams per day — Robert Cathcart's protocol known as a "vitamin C flush" wherein escalating doses of vitamin C are given until diarrhoea develops, then choosing the highest dose that does not cause diarrhea (the bowel tolerance threshold)<;ref name="Cathcart"/>
Vitamin C as a macronutrient
There is a strong advocacy movement for large doses of vitamin C, promoting a great deal of added benefits. Many pro-vitamin C organizations promote usage levels well beyond the current Dietary Reference Intake. The movement is led by scientists and doctors such as Robert Cathcart, Ewan Cameron, Steve Hickey, Irwin Stone and the twice Nobel Prize laureate Linus Pauling and the more controversial Matthias Rath. There is an extensive and growing scientific literature critical of governmental agency dose recommendations.[41][49] The biological halflife for vitamin C is fairly short, about 30 minutes in blood plasma, a fact which high dose advocates say that mainstream researchers have failed to take into account. Researchers at the National Institutes of Health decided upon the current RDA based upon tests conducted 12 hours (24 half lives) after consumption. Hickey, on this matter, says "To be blunt, the NIH gave a dose of vitamin C, waited until it had been excreted, and then measured blood levels."[50]
Evolutionary rationales
Humans carry a mutated and ineffective form of the gene required by all mammals for manufacturing the fourth of the four enzymes that manufacture vitamin C.[51] The inability to produce vitamin C, hypoascorbemia, is, according to the Online Mendeleian Inheritance in Man database, a "public" inborn error of metabolism. The gene, Pseudogene ΨGULO, lost its function millions of years ago, when the anthropoids branched out.[52] In humans the three functional enzymes continue to produce the precursors to vitamin C, but the process is incomplete; these enzymes ultimately undergo proteolytic degradation. Stone[53] and Pauling[40] calculated, based on the diet of our primate cousins[39] (similar to what our common descents are likely to have consumed when the gene mutated), that the optimum daily requirement of vitamin C is around 2300 milligrams for a human requiring 2500 kcal a day.
The established RDA has been criticised by Pauling to be one that will prevent acute scurvy, and is not necessarily the dosage for optimal health.[48]
The controversial Matthias Rath hypothesised that during the ice age, when vitamin C was scarce, natural selection favoured human individuals who could repair arteries with a layer of cholesterol. He suggests that although eventually harmful, cholesterol lining of artery walls would be beneficial in that it would keep the individual alive until access to vitamin C allowed arterial damage to be repaired. If this is true, atherosclerosis is in fact a vitamin C deficiency disease. As atherosclerosis is the main cause of ischaemic heart disease, which in turn is the leading cause of death in developed countries,[54] this would have a profound effect on western medicine.
Therapeutic uses
Since its discovery vitamin C has been considered by some enthusiastic proponents a "universal panacea", although this led to suspicions by others of it being over-hyped.[55] Other proponents of high dose vitamin C consider that if it is given "in the right form, with the proper technique, in frequent enough doses, in high enough doses, along with certain additional agents and for a long enough period of time,"[56] it can prevent and, in many cases, cure, a wide range of common and/or lethal diseases, notably the common cold and heart disease,[57] although the NIH considers there to be "fair scientific evidence against this use."[58] Some proponents issued controversial statements involving it being a cure for AIDS,[59] bird flu, and SARS.[60][61][62]
Probably the most controversial issue, the putative role of ascorbate in the management of AIDS, is still unresolved, more than 16 years after the landmark study published in the prestigious Proceedings of National Academy of Sciences (USA) showing that non toxic doses of ascorbate suppress HIV replication in vitro.[63] Other studies expanded on those results, but still, no large scale trials have yet been conducted.[64][65][66]
A 1986 study indicates that vitamin C may be important in regulation of endogenous cholesterol synthesis.[67]
There have been studies suggesting that vitamin C detoxifies lead,[68][69] reduces the severity of symptoms in children with autism,[70] reduces multiple organ failure and length of stay in the intensive care unit in trauma victims,[71] improves sperm count, sperm motility, and sperm morphology in infertile men,[72] and improves immune function in aged persons and could contribute to the prevention and treatment of age-associated diseases.[73] Dehydroascorbic acid, a chemical relative of Vitamin C but distinct from the chemical itself, was shown to reduce neurological deficits and mortality following stroke, although "the antioxidant ascorbic acid (AA) or vitamin C does not penetrate the blood-brain barrier".[74]
In January 2007 the US Food and Drug Administration approved a new trial of intravenous vitamin C as a cancer treatment for "patients who have exhausted all other conventional treatment options." Additional studies over several years would be needed to demonstrate whether it is effective.[75]
Testing for ascorbate levels in the body
Simple tests use DCPIP to measure the levels of vitamin C in the urine and in serum or blood plasma. However these reflect recent dietary intake rather than the level of vitamin C in body stores.[5] Reverse phase high performance liquid chromatography is used for determining the storage levels of vitamin C within lymphocytes and tissue.
It has been observed that while serum or blood plasma levels follow the circadian rhythm or short term dietary changes, those within tissues themselves are more stable and give a better view of the availability of ascorbate within the organism. However, very few hospital laboratories are adequately equipped and trained to carry out such detailed analyses, and require samples to be analyzed in specialized laboratories.[76][77]
Adverse effects
While being harmless in most typical quantities, as with all substances to which the human body is exposed, vitamin C can still cause harm under certain conditions.
Common side-effects
Relatively large doses of vitamin C may cause indigestion, particularly when taken on an empty stomach. This unpleasant but harmless side-effect can be avoided by taking the vitamin along with meals or by offsetting its acidity by taking an antacid such as baking soda or calcium carbonate.
When taken in huge doses, vitamin C causes diarrhea. The minimum dose that brings about this effect varies with the individual. Robert Cathcart has called this limit the "bowel tolerance threshold" and observed that it is higher in people with serious illness than those in good health.[42] It ranges from 5 to 25 grams per day in healthy individuals to 300 grams per day in those that are severely ill. Diarrhea is not harmful, as long as the dose is reduced quickly.
In one trial, doses up to 6 grams of ascorbic acid were given to 29 infants, 93 children of preschool and school age, and 20 adults for more than 1400 days. With the higher doses, toxic manifestations were observed in five adults and four infants. The signs and symptoms in adults were nausea, vomiting, diarrhea, flushing of the face, headache, fatigue and disturbed sleep. The main toxic reactions in the infants were skin rashes.[78]
Rare side-effects
As vitamin C enhances iron absorption, iron poisoning can become an issue to people with rare iron overload disorders, such as haemochromatosis. A genetic condition that results in inadequate levels of the enzyme glucose-6-phosphate dehydrogenase (G6PD), can cause sufferers to develop hemolytic anemia after ingesting specific oxidizing substances, such as very large dosages of vitamin C. However, there is a test available for G6PD deficiency,[79] and it has been proposed that high doses of vitamin E may protect against this problem.
In addition, large doses of vitamin C (2 g per day) trigger oxalate formation and increase absorption of dietary oxalate, possibly causing kidney stones[1].
Chance of overdose
As discussed previously, vitamin C exhibits remarkably low toxicity. The LD50 (the dose that will kill 50% of a population) in rats is generally accepted to be 11900 milligrams per kilogram when taken orally.[80] The LD50 in humans remains unknown, owing to medical ethics that preclude experiments which would put patients at risk of harm. However , as with all substances tested in this way, the LD50 is taken as a guide to its toxicity in humans and no data to contradict this has been found.
Natural and artificial dietary sources
The richest natural sources are fruits and vegetables, and of those, the camu camu fruit and the Kakadu plum contain the highest concentration of the vitamin. It is also present in some cuts of meat, especially liver. Vitamin C is the most widely taken nutritional supplement and is available in a variety of forms, including tablets, drink mixes, crystals in capsules or naked crystals.
Plant sources
While plants are generally a good source of vitamin C, the amount in foods of plant origin depends on: the precise variety of the plant, the soil condition, the climate in which it grew, the length of time since it was picked, the storage conditions, and the method of preparation.[81]
The following table is approximate and shows the relative abundance in different raw plant sources.[82][83][84] The amount is given in milligrams per 100 grams of fruit or vegetable and is a rounded average from multiple authoritative sources:
| Plant source | Amount (mg / 100g) |
|---|---|
| Kakadu plum | 3150 |
| Camu Camu | 2800 |
| Rose hip | 2000 |
| Acerola | 1600 |
| Amla | 720 |
| Jujube | 500 |
| Baobab | 400 |
| Blackcurrant | 200 |
| Red pepper | 190 |
| Parsley | 130 |
| Seabuckthorn | 120 |
| Guava | 100 |
| Kiwifruit | 90 |
| Broccoli | 90 |
| Loganberry | 80 |
| Redcurrant | 80 |
| Brussels sprouts | 80 |
| Lychee | 70 |
| Cloudberry | 60 |
| Persimmon | 60 |
| Plant source | Amount (mg / 100g) |
|---|---|
| Papaya | 60 |
| Strawberry | 60 |
| Orange | 50 |
| Lemon | 40 |
| Melon, cantaloupe | 40 |
| Cauliflower | 40 |
| Grapefruit | 30 |
| Raspberry | 30 |
| Tangerine | 30 |
| Mandarin orange | 30 |
| Passion fruit | 30 |
| Spinach | 30 |
| Cabbage raw green | 30 |
| Lime | 20 |
| Mango | 20 |
| Potato | 20 |
| Melon, honeydew | 20 |
| Mango | 16 |
| Tomato | 10 |
| Blueberry | 10 |
| Pineapple | 10 |
| Plant source | Amount (mg / 100g) |
|---|---|
| Pawpaw | 10 |
| Grape | 10 |
| Apricot | 10 |
| Plum | 10 |
| Watermelon | 10 |
| Banana | 9 |
| Carrot | 9 |
| Avocado | 8 |
| Crabapple | 8 |
| Peach | 7 |
| Apple | 6 |
| Blackberry | 6 |
| Beetroot | 5 |
| Pear | 4 |
| Lettuce | 4 |
| Cucumber | 3 |
| Eggplant | 2 |
| Fig | 2 |
| Bilberry | 1 |
| Horned melon | 0.5 |
| Medlar | 0.3 |
Animal sources
The overwhelming majority of species of animals and plants synthesise their own vitamin C, making some, but not all, animal products, sources of dietary vitamin C.
Vitamin C is most present in the liver and least present in the muscle. Since muscle provides the majority of meat consumed in the western human diet, animal products are not a reliable source of the vitamin. Vitamin C is present in mother's milk and, in lower amounts, in raw cow's milk, with pasteurized milk containing only trace amounts.[85] All excess Vitamin C is disposed of through the urinary system.
The following table shows the relative abundance of vitamin C in various foods of animal origin, given in milligram of vitamin C per 100 grams of food:
| Food | Amount (mg / 100g) |
|---|---|
| Calf liver (raw) | 36 |
| Beef liver (raw) | 31 |
| Oysters (raw) | 30 |
| Cod roe (fried) | 26 |
| Pork liver (raw) | 23 |
| Lamb brain (boiled) | 17 |
| Chicken liver (fried) | 13 |
| Lamb liver (fried) | 12 |
| Lamb heart (roast) | 11 |
| Food | Amount (mg / 100g) |
|---|---|
| Lamb tongue (stewed) | 6 |
| Human milk (fresh) | 4 |
| Goat milk (fresh) | 2 |
| Cow milk (fresh) | 2 |
| Beef steak (fried) | 0 |
| Hen's egg (raw) | 0 |
| Pork bacon (fried) | 0 |
| Calf veal cutlet (fried) | 0 |
| Chicken leg (roast) | 0 |
Food preparation
Vitamin C chemically decomposes under certain conditions, many of which may occur during the cooking of food. Normally, boiling water at 100°C is not hot enough to cause any significant destruction of the nutrient, which only decomposes at 190°C, despite popular opinion. However, pressure cooking, roasting, frying and grilling food is more likely to reach the decomposition temperature of vitamin C. Longer cooking times also add to this effect, as will copper food vessels, which catalyse the decomposition.[34]
Another cause of vitamin C being lost from food is leaching, where the water-soluble vitamin dissolves into the cooking water, which is later poured away and not consumed. However, vitamin C doesn't leach in all vegetables at the same rate; research shows broccoli seems to retain more than any other.[86] Research has also shown that fresh-cut fruit don't lose significant nutrients when stored in the refrigerator for a few days.[87]
Vitamin C supplements
Vitamin C is the most widely taken dietary supplement.[88] It is available in many forms including caplets, tablets, capsules, drink mix packets, in multi-vitamin formulations, in multiple antioxidant formulations, as chemically pure crystalline powder, timed release versions, and also including bioflavonoids such as quercetin, hesperidin and rutin. The use of vitamin C supplements with added bioflavonoids and, often, flavours and sweeteners, can be problematic at gram dosages, since those additives are not so well studied as vitamin C. Also, the presence of glucose in the intestines or bloodstream inhibits the absorption of vitamin C. Tablet and capsule sizes range from 25 mg to 1500 mg. Vitamin C (as ascorbic acid) crystals are typically available in bottles containing 300 g to 1 kg of powder (a teaspoon of vitamin C crystals equals 5,000 mg). In supplements, vitamin C most often comes in the form of various mineral ascorbates, as they are easier to absorb, more easily tolerated and provide a source of several dietary minerals.
Absorption of Vitamin C
Vitamin C is absorbed by the intestines using a sodium-ion dependent channel. It is transported through the intestine via both glucose-sensitive and glucose-insensitive mechanisms. Having a lot of sugar either in your intestines or in your blood (as in diabetes mellitus) can slow absorption, which is relevant when megadosing.[89]
Artificial modes of synthesis
Vitamin C is produced from glucose by two main routes. The Reichstein process, developed in the 1930s, uses a single pre-fermentation followed by a purely chemical route. The modern two-step fermentation process, originally developed in China in the 1960s, uses additional fermentation to replace part of the later chemical stages. Both processes yield approximately 60% vitamin C from the glucose feed.[90]
Research is underway at the Scottish Crop Research Institute in the interest of creating a strain of yeast that can synthesise vitamin C in a single fermentation step from galactose, a technology expected to reduce manufacturing costs considerably.[29]
World production of synthesised vitamin C is currently estimated at approximately 110,000 tonnes annually. Main producers today are BASF/Takeda, DSM, Merck and the China Pharmaceutical Group Ltd. of the People's Republic of China. China is slowly becoming the major world supplier as its prices undercut those of the US and European manufacturers.[91]
ReferencesISBN links support NWE through referral fees
- ↑ 1.0 1.1 Vitamin C. Food Standards Agency (UK). Retrieved 2007-02-19.
- ↑ Vitamin C (Ascorbic Acid). University of Maryland Medical Center (April 2002). Retrieved 2007-02-19.
- ↑ 3.0 3.1 Higdon, Jane, Ph.D. (2006-01-31). Vitamin C. Oregon State University, Micronutrient Information Center. Retrieved 2007-03-07.
- ↑ Padayatty S, Katz A, Wang Y, Eck P, Kwon O, Lee J, Chen S, Corpe C, Dutta A, Dutta S, Levine M (2003). Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22 (1): 18-35.
- ↑ 5.0 5.1 5.2 5.3 5.4 Vitamin C – Risk Assessment. UK Food Standards Agency. Retrieved 2007-02-19.
- ↑ Joseph Louis Svirbelf, Albert Szent-Gyorgyi The Chemical Nature Of Vitamin C, April 25th, 1932. Part of the National Library of Medicine collection. Accessed January 2007
- ↑ Meister A (1994). Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem 269 (13): 9397-400.
- ↑ Levine M, Rumsey SC, Wang Y, Park JB, Daruwala R. Vitamin C. In Stipanuk MH (ed): "Biochemical and Physiological Aspects of Human Nutrition." Philadelphia: W B Saunders, pp 541–567, 2000.
- ↑ Prockop DJ, Kivirikko KI: Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem 64:403–434, 1995.
- ↑ Peterkofsky B: Ascorbate requirement for hydroxylation and secretion of procollagen: relationship to inhibition of collagen synthesis in scurvy. Am J Clin Nutr 54:1135S–1140S, 1991.
- ↑ Kivirikko KI, Myllyla R: Post-translational processing of procollagens. Ann N Y Acad Sci 460:187–201, 1985.
- ↑ McGee, William, M.D., M.H.A., Assistant Professor of Medicine and Surgery, Tufts University School of Medicine; Medical Encyclopedia: Ascorbic acid
- ↑ Rebouche CJ: Ascorbic acid and carnitine biosynthesis. Am J Clin Nutr 54:1147S–1152S, 1991.
- ↑ Dunn WA, Rettura G, Seifter E, Englard S. Carnitine biosynthesis from gamma-butyrobetaine and from exogenous protein-bound 6-N-trimethyl-L-lysine by the perfused guinea pig liver. Effect of ascorbate deficiency on the in situ activity of gammabutyrobetaine hydroxylase. J Biol Chem 259:10764–10770, 1984.
- ↑ Levine M, Dhariwal KR, Washko P, Welch R, Wang YH, Cantilena CC, Yu R: Ascorbic acid and reaction kinetics in situ: a new approach to vitamin requirements. J Nutr Sci Vitaminol (Tokyo) Spec No:169–172, 1992.
- ↑ Kaufman S: Dopamine-beta-hydroxylase. J Psychiatr Res 11: 303–316, 1974.
- ↑ Eipper BA, Milgram SL, Husten EJ, Yun HY, Mains RE: Peptidylglycine alpha-amidating monooxygenase: a multifunctional protein with catalytic, processing, and routing domains. Protein Sci 2:489–497, 1993.
- ↑ Eipper BA, Stoffers DA, Mains RE: The biosynthesis of neuropeptides: peptide alpha-amidation. Annu Rev Neurosci 15:57–85, 1992.
- ↑ Englard S, Seifter S (1986). The biochemical functions of ascorbic acid. Annu. Rev. Nutr. 6: 365-406.
- ↑ Lindblad B, Lindstedt G, Lindstedt S: The mechanism of enzymic formation of homogentisate from p-hydroxyphenylpyruvate. J Am Chem Soc 92:7446–7449, 1970.
- ↑ New view at C Matthias A. Hediger , Nature Medicine 8, 445 - 446 (2002)
doi:10.1038/nm0502-445
"Plasma vitamin C concentrations are maintained between 10 and 160 μM, and any excess of the vitamin is excreted by the kidney. In contrast, the vitamin is concentrated to at least 100 times the plasma level in specific tissues such as the adrenal glands, pituitary gland, thymus, retina, corpus luteum, and a variety of neuronal cell types."
- ↑ Bánhegyi G, Mándl J (2001). The hepatic glycogenoreticular system. Pathol Oncol Res 7 (2): 107-10.
- ↑ Harris, J. Robin (1996). Ascorbic Acid: Subcellular Biochemistry. Springer, p. 35. ISBN 0306451484.
- ↑ Milton, K. (1999) "Nutritional characteristics of wild primate foods: do the diets of our closest living relatives have lessons for us?" Nutrition. 1999 Jun;15(6):488-98.
- ↑ Proctor P (1970). Similar functions of uric acid and ascorbate in man?. Nature 228 (5274): 868.
- ↑ Stone, Irwin (July 16, 1978). Eight Decades of Scurvy. The Case History of a Misleading Dietary Hypothesis. Retrieved 2007-04-06.
- ↑ Linster, Carole; Emile Van Schaftingen (December 12, 2006). Vitamin C: Biosynthesis, recycling and degradation in mammals. Retrieved 2007-04-30.
- ↑ C. Long, et al.. Ascorbic acid dynamics in the seriously ill and injured.. Journal of Surgical Research 109 (2): 144–148.
"Our results show that plasma ascorbic acid levels following trauma and during infection are extremely low and are not normalized with 300 or even 1000 mg/day supplemented TPN."
- ↑ 29.0 29.1 R.D. Hancock & R. Viola. Ascorbic acid biosynthesis in higher plants and micro-organisms. Scottish Crop Research Institute. Retrieved 2007-02-20.
"Our results demonstrate that yeast cells are capable of direct fermentation of L-galactose to L-AA. However, given that L-galactose is an extremely rare and expensive sugar a process using L-galactose as a starting material could never be economical. In order to overcome this problem, we are currently developing new yeast strains with extended metabolic competence for the synthesis of L-galactose directly from inexpensive substrates."
- ↑ Hancock RD, Galpin JR, Viola R.. Biosynthesis of L-ascorbic acid (vitamin C) by Saccharomyces cerevisiae. FEMS Microbiol Lett. 186 (2): 245-50.
- ↑ McGee, William (2007-01-02). Vitamin C. National Institutes of Health. Retrieved 2007-03-09.
- ↑ Jacques Cartier's Second Voyage - 1535 - Winter & Scurvy. Retrieved 2007-02-25.
- ↑ Martini E. (June 2002). Jacques Cartier witnesses a treatment for scurvy. Vesalius.
- ↑ 34.0 34.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedoxford - ↑ Pitt History - 1932: Charles Glen King. University of Pittsburgh. Retrieved 2007-02-21.
- ↑ Burns, J. J., and Evans, C., J. Biol. Chem., 200, 125 (1953).
- ↑ Burns, J. J., Peyser, P., and Maltz, A., Science, 124, 1148 (1956).
- ↑ 38.0 38.1 38.2 38.3 US Recommended Dietary Allowance (RDA). Retrieved 2007-02-19.
- ↑ 39.0 39.1 Milton K (2003). Micronutrient intakes of wild primates: are humans different?. Comp Biochem Physiol A Mol Integr Physiol 136 (1): 47-59.
- ↑ 40.0 40.1 Pauling, Linus. Evolution and the need for ascorbic acid. Proc Natl Acad Sci U S A 67 (4): 1643-8.
- ↑ 41.0 41.1 Linus Pauling Vindicated; Researchers Claim RDA For Vitamin C is Flawed. PR Newswire (6 July 2004). Retrieved 2007-02-20.
- ↑ 42.0 42.1 42.2 Cathcart, Robert (1994). Vitamin C, Titrating To Bowel Tolerance, Anascorbemia, and Acute Induced Scurvy. Orthomed. Retrieved 2007-02-22.
- ↑ Vitamin and mineral requirements in human nutrition, 2nd edition. World Health Organization (2004). Retrieved 2007-02-20.
- ↑ Higdon, Jane. Linus Pauling Institute Recommendations. Oregon State University. Retrieved 2007-04-11.
- ↑ Roc Ordman. The Scientific Basis Of The Vitamin C Dosage Of Nutrition Investigator. Beloit College. Retrieved 2007-02-22.
- ↑ Vitamin C Foundation's RDA. Retrieved 2007-02-12.
- ↑ Levy, Thomas E. (2002). Vitamin C Infectious Diseases, & Toxins. Xlibris. ISBN 1401069630. Chapter 5 - Vitamin C optidosing.
- ↑ 48.0 48.1 Pauling, Linus (1986). How to Live Longer and Feel Better. W. H. Freeman and Company. ISBN 0-380-70289-4.
- ↑ Forman, Robert (1981). Medical Resistance To Innovation. Medical Hypotheses 7 (8): 1009-1017.
- ↑ Sardi, Bill (July 09, 2004). The Vitamin C Fanatics Were Right All Along. Knowledge of Health, Inc.. Retrieved 2007-02-22.
- ↑ OMIM. Online Mendeleian Inheritance in Man. HYPOASCORBEMIA.
- ↑ Hickey, Steve and Roberts, Hilary (2004). Ascorbate: The Science of Vitamin C. Lulu Press, Inc.. ISBN 1-4116-0724-4.
- ↑ Stone, Irwin (1972). The Healing Factor: Vitamin C Against Disease. Grosset and Dunlap. ISBN 0-448-11693-6.
- ↑ WHO World Health Report 2002
- ↑ Hemilä, Harri (January 2006). Do vitamins C and E affect respiratory infections? (PDF). University of Helsinki. Retrieved 2007-02-21.
- ↑ Levy, Thomas E. (2002). Curing the Incurable: Vitamin C, Infectious Diseases, and Toxins. Livon Books, p. 36. ISBN 1-4010-6963-0.
- ↑ Rath MW, Pauling LC. U.S. Patent 5278189 (PDF) Prevention and treatment of occlusive cardiovascular disease with ascorbate and substances that inhibit the binding of lipoprotein(a). USPTO. 11 Jan 1994.
- ↑ http://www.nlm.nih.gov/medlineplus/druginfo/natural/patient-vitaminc.html
- ↑ Nigeria: Vitamin C Can Suppress HIV/Aids Virus. allAfrica.com (2006-05-22). Retrieved 2006-06-16.
- ↑ Hemilä H (2003). Vitamin C and SARS coronavirus. J Antimicrob Chemother 52 (6): 1049-50.
- ↑ Boseley, Sarah. "Discredited doctor's 'cure' for Aids ignites life-and-death struggle in South Africa", The Guardian, 2005-05-14. Retrieved 2007-02-21.
- ↑ Rath, Matthias (2005). Open letter from Dr. Matthias Rath MD to German Chancellor Merkel. Dr. Rath Health Foundation. Retrieved 2007-02-21.
- ↑ Harakeh S, Jariwalla R, Pauling L (1990). Suppression of human immunodeficiency virus replication by ascorbate in chronically and acutely infected cells. Proc Natl Acad Sci U S A 87 (18): 7245-9.
- ↑ Harakeh S, Jariwalla R (1991). Comparative study of the anti-HIV activities of ascorbate and thiol-containing reducing agents in chronically HIV-infected cells. Am J Clin Nutr 54 (6 Suppl): 1231S-1235S.
- ↑ Harakeh S, Jariwalla R (1997). NF-kappa B-independent suppression of HIV expression by ascorbic acid. AIDS Res Hum Retroviruses 13 (3): 235-9.
- ↑ Harakeh S, Jariwalla R. Ascorbate effect on cytokine stimulation of HIV production. Nutrition 11 (5 Suppl): 684-7.
- ↑ Harwood H, Greene Y, Stacpoole P (1986). Inhibition of human leukocyte 3-hydroxy-3-methylglutaryl coenzyme A reductase activity by ascorbic acid. An effect mediated by the free radical monodehydroascorbate. J Biol Chem 261 (16): 7127-35.
- ↑ Harry N. Holmes, Kathryn Campbell, Edward J. Amberg. The Effect of Vitamin C on Lead Poisoning. AscorbateWeb. Retrieved 2007-02-19.
- ↑ Dawson E, Evans D, Harris W, Teter M, McGanity W (1999). The effect of ascorbic acid supplementation on the blood lead levels of smokers. J Am Coll Nutr 18 (2): 166-70.
- ↑ Baxter, Peter (2002). Vitamin Responsive Conditions in Paediatric Neurology. MacKeith Press, p. 24. ISBN 189868328X.
- ↑ Nathens A, Neff M, Jurkovich G, Klotz P, Farver K, Ruzinski J, Radella F, Garcia I, Maier R (2002). Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Ann Surg 236 (6): 814-22.
- ↑ Akmal M, Qadri J, Al-Waili N, Thangal S, Haq A, Saloom K (2006). Improvement in human semen quality after oral supplementation of vitamin C. J Med Food 9 (3): 440-2.
- ↑ de la Fuente M, Ferrández M, Burgos M, Soler A, Prieto A, Miquel J (1998). Immune function in aged women is improved by ingestion of vitamins C and E. Can J Physiol Pharmacol 76 (4): 373-80.
- ↑ Huang J, Agus DB, Winfree CJ, Kiss S, Mack WJ, McTaggart RA, Choudhri TF, Kim LJ, Mocco J, Pinsky DJ, Fox WD, Israel RJ, Boyd TA, Golde DW, Connolly ES Jr. (2001). Dehydroascorbic acid, a blood-brain barrier transportable form of vitamin C, mediates potent cerebroprotection in experimental stroke. Proceedings of the National Academy of Sciences 98 (20): 11720-11724.
- ↑ FDA OKs vitamin C trial for cancer. Physorg.com (January 12, 2007). Retrieved 2007-04-06.
- ↑ Emadi-Konjin P, Verjee Z, Levin A, Adeli K (2005). Measurement of intracellular vitamin C levels in human lymphocytes by reverse phase high performance liquid chromatography (HPLC).. Clinical Biochemistry 38 (5): 450-6. PMID 15820776.
"Serum and plasma vitamin C measurements do not correlate well with tissue levels while lymphocyte vitamin C levels provide the most accurate assessment of the true status of vitamin C stores and are not affected acutely by circadian rhythm or dietary changes."
- ↑ Yamada H, Yamada K, Waki M, Umegaki K. (2004). Lymphocyte and Plasma Vitamin C Levels in Type 2 Diabetic Patients With and Without Diabetes Complications. Diabetes Care 27: 2491–2.
"The plasma concentration of vitamin C is considered to be strongly correlated with transient consumption of foods. The measurement of lymphocyte vitamin C might be expected to be a more reliable antioxidant biomarker than plasma vitamin C level. In this report, we demonstrated that the lymphocyte vitamin C level is significantly lower in type 2 diabetic patients, but we could not observe such an association in plasma vitamin C levels. In diabetes, therefore, the measurement of lymphocyte vitamin C might be expected to be a more reliable antioxidant biomarker than plasma vitamin C level."
- ↑ Toxicological evaluation of some food additives including anticaking agents, antimicrobials, antioxidants, emulsifiers and thickening agents. World Health Organization (4 July 1973). Retrieved 2007-04-13.
- ↑ Intravenous Ascorbate as a Chemotherapeutic and Biologic Response Modifying Agent. The Center For The Improvement Of Human Functioning International. Retrieved 2007-02-19.
- ↑ Safety (MSDS) data for ascorbic acid. Oxford University (2005-10-09). Retrieved 2007-02-21.
- ↑ The vitamin and mineral content is stable. Danish Veterinary and Food Administration. Retrieved 2007-03-07.
- ↑ National Nutrient Database. Nutrient Data Laboratory of the US Agricultural Research Service. Retrieved 2007-03-07.
- ↑ Vitamin C Food Data Chart. Healty Eating Club. Retrieved 2007-03-07.
- ↑ Natural food-Fruit Vitamin C Content. The Natural Food Hub. Retrieved 2007-03-07.
- ↑ Clark, Stephanie, Ph.D (8 January 2007). Comparing Milk: Human, Cow, Goat & Commercial Infant Formula. Washington State University. Retrieved 2007-02-28.
- ↑ Combs GF. The Vitamins, Fundamental Aspects in Nutrition and Health. 2nd ed. San Diego, CA: Academic Press, 2001:245–272
- ↑ Hitti, Miranda (2 June 2006). Fresh-Cut Fruit May Keep Its Vitamins. WebMD. Retrieved 2007-02-25.
- ↑ The Diet Channel Vitamin C might be the most widely known and most popular vitamin purchased as a supplement.
- ↑ Wilson JX (2005). Regulation of vitamin C transport. Annu. Rev. Nutr. 25: 105-25.
- ↑ The production of vitamin C. Competition Commission (2001). Retrieved 2007-02-20.
- ↑ Patton, Dominique (2005-10-20). DSM makes last stand against Chinese vitamin C. nutraingredients. Retrieved 2007-02-20.
Further reading
- Journals
- Dolske, M.C., et al. (1993). A preliminary trial of ascorbic acid as supplemental therapy for autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 17 (5): 765-74.
- Green VA, Pituch KA, Itchon J, Choi A, O'Reilly M, Sigafoos J (2006). Internet survey of treatments used by parents of children with autism. Research in developmental disabilities 27 (1): 70-84.
- Books
- Pauling, Linus (1970). Vitamin C and the Common Cold. W. H. Freeman & Company. ISBN 071670160X.
- Pauling, Linus (1976). Vitamin C, the Common Cold, and the Flu. W H Freeman & Co. ISBN 0716703610.
- Cameron, Ewan and Linus Pauling, (1979). Cancer and Vitamin C. Pauling Institute of Science and Medicine. ISBN 0393500004.
- Kent, Saul (1980). Life Extension Revolution. Morrow.
- Pearson, Durk and Sandy Shaw (1982). Life Extension: A Practical Scientific Approach. Warner Books. ISBN 0446387355. see Part IV, Chapter 7: Vitamin C
- Pelton, Ross (1986). Mind Food and Smart Pills: How to Increase Your Intelligence and Prevent Brain Aging. T & R Pub. ISBN 0936809000. see Chapter 3: Vitamin C, The Champion Free Radical Scavenger
- Clemetson, C.A.B (1989). Vitamin C. Boca Raton, Florida: CRC Press. ISBN 0-8493-4841-2. Monograph - Volumes I, II, III.
- Levy, Thomas E. (2002). Vitamin C Infectious Diseases, & Toxins. Xlibris. ISBN 1401069630.
External links
- Jane Higdon, "Vitamin C", Micronutrient Information Center, Linus Pauling Institute
- AscorbateWeb — a collection of twentieth century medical & scientific literature on vitamin C in the treatment and prevention of human disease at seanet.com
- Healing Thresholds — Research on Vitamin C in the treatment of autism. at healingthresholds.com
- U.S. Patent 5278189 (PDF) — "Prevention and treatment of occlusive cardiovascular disease with ascorbate and substances that inhibit the binding of lipoprotein (a)", Inventors: Matthias W. Rath and Linus C. Pauling
- vitamin C at United Kingdom Food Standards Agency
- Naidu KA (2003). Vitamin C in human health and disease is still a mystery? An overview. Nutrition journal 2: 7.
- For Doctors: Preparation of Vitamin C IV's — by Andrew W. Saul, PhD. at doctoryourself.com
- Information regarding treatment of the Bird Flu with massive doses of ascorbate. — by Robert Cathcart, M.D. at orthomed.com
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.