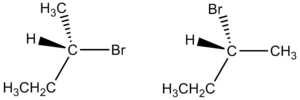
Enantiomer
In chemistry, an enantiomer (from the Greek words á¼Î½Î¬Î½ÏιοÏ, meaning "opposite," and μÎÏοÏ, meaning "part" or "portion") is one of two stereoisomers that are nonsuperimposable complete mirror images of each other.[1] The situation is analogous to a person's left and right hands, which are nonsuperimposable mirror images. A compound is said to be enantiomerically pure, or enantiopure, if it consists of molecules of only one chirality, within the limits of detectability.[2] Enantiomers have important functions in living organisms.
Overall properties
When present in a symmetric environment, enantiomers have identical chemical and physical properties, except for their ability to rotate plane-polarized light by equal amounts but in opposite directions. For this reason, enantiomers are also called "optical isomers." A mixture of equal parts of an optically active isomer and its enantiomer is termed racemic and has a net rotation of plane-polarized light of zero.
Two symmetrical enantiomers often do have different chemical properties that are revealed when they interact with other substances that are also enantiomers. Because many molecules in the bodies of living beings are enantiomers themselves, there is often a marked difference in the effects of two symmetrical enantiomers on living beings, including human beings.
Enantioselective preparations
There are two main strategies for the preparation of enantiopure compounds. The first is known as chiral resolution. This method involves preparing the compound in racemic form, and separating it into its isomers. In his pioneering work, Louis Pasteur was able to isolate the isomers of tartaric acid because they crystallize from solution as crystals each with a different symmetry. A less common method is by enantiomer self-disproportionation.
The second strategy is asymmetric synthesis: The use of various techniques to prepare the desired compound in high enantiomeric excess. Techniques encompassed include the use of chiral starting materials (chiral pool synthesis), the use of chiral auxiliaries and chiral catalysts, and the application of asymmetric induction. The use of enzymes (biocatalysis) may also produce the desired compound.
Enantioconvergent synthesis is the synthesis of one enantiomer from a racemic precursor molecule utilizing both enantiomers.
Enantiopure medications
Advances in industrial chemical processes have made it economical for pharmaceutical manufacturers to take drugs that were originally marketed in racemic form and market the individual enantiomers, each of which may have unique properties. For some drugs, such as zopiclone, only one enantiomer (eszopiclone) is active; the FDA has allowed such once-generic drugs to be patented and marketed under another name.[3]. In other cases, such as ibuprofen, it is not economically feasible to isolate a single enantiomer from a racemic mixture or to synthesize just the active one, and therefore a racemic mixture is marketed, with an essentially doubled recommended dose.
Examples of racemic mixtures and the corresponding single-enantiomer products that have been marketed include:
- Amphetamine (Benzedrine; street amphetamine is also racemic) and dextroamphetamine (Dexedrine)
- Bupivacaine (Marcain) and levobupivacaine (Chirocaine)
- Cetirizine (Zyrtec / Reactine) and levocetirizine (Xyzal)
- Citalopram (Celexa / Cipramil) and escitalopram (Lexapro / Cipralex)
- Methylphenidate (Ritalin) and dexmethylphenidate (Focalin)
- Modafinil (Provigil) and armodafinil (Nuvigil)
- Ofloxacin (Floxin) and levofloxacin (Levaquin)
- Omeprazole (Prilosec) and esomeprazole (Nexium)
- Salbutamol (Ventolin) and levalbuterol (Xopenex)
- Zopiclone (Imovane) and eszopiclone (Lunesta)
Thalidomide is an example of a racemic drug, in which one enantiomer produces a desirable antiemetic effect, whereas the other is toxic and produces a teratogenic side-effect. However, the enantiomers are converted into each other in vivo, so chemical processes cannot be used to mitigate its toxicity.
See also
- Chirality (chemistry)
- Isomer
- Organic chemistry
- Stereochemistry
Notes
- â IUPAC Gold Book, enantiomer. Retrieved September 25, 2008.
- â IUPAC, Gold Book, enantiomerically pure (enantiopure). Retrieved September 25, 2008.
- â U.S. Food and Drug Administration, FDA's policy statement for the development of new stereoisomeric drugs. Retrieved September 25, 2008.
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry, 6th edition. Thomson, 2004. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0-13-643669-2.
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley, 2003. ISBN 0471417998.
External links
All links retrieved February 13, 2024.
| ||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.