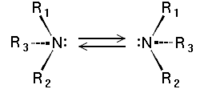Chirality (chemistry)
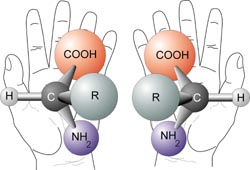
The term chiral is used to describe an object that is not superposable on its mirror image. Human hands are perhaps the most universally recognized example of chirality: The left hand is not a superposable mirror image of the right hand; no matter how the two hands are oriented, it is impossible for all the major features of both hands to coincide.
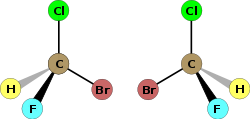
In the context of chemistry, chirality usually refers to molecules. Two mirror images of a molecule that cannot be superposed on each other are referred to as enantiomers or optical isomers. Because the difference between right and left hands is universally known and easy to observe, many pairs of enantiomers are designated as "right-" and "left-handed." A mixture of equal amounts of the two enantiomers is said to be a racemic mixture. Racemic mixtures are not optically active even though their two enantiomer constituents when separated are. Molecular chirality is of interest because of its application to stereochemistry in inorganic chemistry, organic chemistry, physical chemistry, biochemistry, and supramolecular chemistry.
The symmetry of a molecule (or any other object) determines whether it is chiral. A molecule is achiral (not chiral) if and only if it has an axis of improper rotation; that is, an n-fold rotation (rotation by 360¬į/n) followed by a reflection in the plane perpendicular to this axis that maps the molecule onto itself. A simplified (if incomplete) rule is that the molecule lacks a plane of symmetry. For tetrahedral molecules, if all four substituents on the central atom are different, the molecule is chiral. A chiral molecule is not necessarily asymmetric, that is, devoid of any symmetry elements, as it can have, for example, rotational symmetry.
Etymology
The term chirality is derived from the Greek word for hand, ŌáőĶőĻŌĀ-(/cheir/).
History
The term optical activity is derived from the interaction of chiral materials with polarized light. A solution of the (‚ąí)-form of an optical isomer rotates the plane of polarization of a beam of plane polarized light in a counterclockwise direction, vice-versa for the (+) optical isomer. The property was first observed by Jean-Baptiste Biot in 1815,[1] and gained considerable importance in the sugar industry, analytical chemistry, and pharmaceuticals. Louis Pasteur deduced in 1848 that this phenomenon has a molecular basis.[2] Artificial composite materials displaying the analog of optical activity but in the microwave region were introduced by J.C. Bose in 1898,[3] and gained considerable attention from the mid-1980s.[4] The term chirality itself was coined by Lord Kelvin in 1873.[5]
The word ‚Äúracemic‚ÄĚ is derived from the Latin word for grape; the term having its origins in the work of Louis Pasteur who isolated racemic tartaric acid from wine.
Naming conventions
By configuration: R- and S-
For chemists, the R / S system is the most important nomenclature system for denoting enantiomers, which does not involve a reference molecule such as glyceraldehyde. It labels each chiral center R or S according to a system by which its substituents are each assigned a priority, according to the Cahn Ingold Prelog priority rules (CIP), based on atomic number. If the center is oriented so that the lowest-priority of the four is pointed away from a viewer, the viewer will then see two possibilities: If the priority of the remaining three substituents decreases in clockwise direction, it is labeled R (for Rectus), if it decreases in counterclockwise direction, it is S (for Sinister).
This system labels each chiral center in a molecule (and also has an extension to chiral molecules not involving chiral centers). Thus, it has greater generality than the D/L system, and can label, for example, an (R,R) isomer versus an (R,S)‚ÄĒdiastereomers.
The R / S system has no fixed relation to the (+)/(‚ąí) system. An R isomer can be either dextrorotatory or levorotatory, depending on its exact substituents.
The R / S system also has no fixed relation to the D/L system. For example, the side-chain one of serine contains a hydroxyl group, -OH. If a thiol group, -SH, were swapped in for it, the D/L labeling would, by its definition, not be affected by the substitution. But this substitution would invert the molecule's R / S labeling, because the CIP priority of CH2OH is lower than that for CO2H but the CIP priority of CH2SH is higher than that for CO2H.
For this reason, the D/L system remains in common use in certain areas of biochemistry, such as amino acid and carbohydrate chemistry, because it is convenient to have the same chiral label for all of the commonly occurring structures of a given type of structure in higher organisms. In the D/L system, they are nearly all consistent‚ÄĒnaturally occurring amino acids are nearly all L, while naturally occurring carbohydrates are nearly all D. In the R / S system, they are mostly S, but there are some common exceptions.
By optical activity: (+)- and (‚ąí)-
An enantiomer can be named by the direction in which it rotates the plane of polarized light. If it rotates the light clockwise (as seen by a viewer towards whom the light is traveling), that enantiomer is labeled (+). Its mirror-image is labeled (‚ąí). The (+) and (‚ąí) isomers have also been termed d- and l-, respectively (for dextrorotatory and levorotatory). This labeling is easy to confuse with D- and L-.
By configuration: D- and L-
An optical isomer can be named by the spatial configuration of its atoms. The D/L system does this by relating the molecule to glyceraldehyde. Glyceraldehyde is chiral itself, and its two isomers are labeled D and L (typically typeset in small caps in published work). Certain chemical manipulations can be performed on glyceraldehyde without affecting its configuration, and its historical use for this purpose (possibly combined with its convenience as one of the smallest commonly used chiral molecules) has resulted in its use for nomenclature. In this system, compounds are named by analogy to glyceraldehyde, which, in general, produces unambiguous designations, but is easiest to see in the small biomolecules similar to glyceraldehyde. One example is the amino acid alanine, which has two optical isomers, and they are labeled according to which isomer of glyceraldehyde they come from. On the other hand, glycine, the amino acid derived from glyceraldehyde, has no optical activity, as it is not chiral (achiral). Alanine, however, is chiral.
The D/L labeling is unrelated to (+)/(‚ąí); it does not indicate which enantiomer is dextrorotatory and which is levorotatory. Rather, it says that the compound's stereochemistry is related to that of the dextrorotatory or levorotatory enantiomer of glyceraldehyde‚ÄĒthe dextrorotatory isomer of glyceraldehyde is, in fact, the D isomer. Nine of the nineteen L-amino acids commonly found in proteins are dextrorotatory (at a wavelength of 589 nm), and D-fructose is also referred to as levulose because it is levorotatory.
A rule of thumb for determining the D/L isomeric form of an amino acid is the "CORN" rule. The groups:
- COOH, R, NH2 and H (where R is a variant carbon chain)
are arranged around the chiral center carbon atom. Sighting with the hydrogen atom away from the viewer, if these groups are arranged clockwise around the carbon atom, then it is the D-form. If counter-clockwise, it is the L-form.
Nomenclature
- Any non-racemic chiral substance is called scalemic[6]
- A chiral substance is enantiopure or homochiral when only one of two possible enantiomers is present.
- A chiral substance is enantioenriched or heterochiral when an excess of one enantiomer is present but not to the exclusion of the other.
- Enantiomeric excess or ee is a measure for how much of one enantiomer is present compared to the other. For example, in a sample with 40 percent ee in R, the remaining 60 percent is racemic with 30 percent of R and 30 percent of S, so that the total amount of R is 70 percent.
Molecules with and without stereogenic centers
In general, chiral molecules have point chirality at a single stereogenic atom, usually carbon, which has four different substituents. The two enantiomers of such compounds are said to have different absolute configurations at this center. This center is thus stereogenic (i.e., a grouping within a molecular entity that may be considered a focus of stereoisomerism), and is exemplified by the őĪ-carbon of amino acids. A molecule can have multiple chiral centers without being chiral overall if there is a symmetry element (a mirror plane or inversion center), which relates the two (or more) chiral centers. Such a molecule is called a meso compound. It is also possible for a molecule to be chiral without having actual point chirality. Common examples include 1,1'-bi-2-naphthol (BINOL) and 1,3-dichloro-allene, which have axial chirality, and (E)-cyclooctene, which has planar chirality.
An undistorted tetrahedral sp3-hybridized carbon atom bearing four freely rotating rigorously identical substituents can be chiral if the substituents themselves are chiral. C(R)4 and C(S)4 are enantiomers without central carbon nomenclature, such as C-[(R)-C(H)(CH3)(OCH3)]4. C(R)3(S) and C(S)3(R) are sufficient. Homochiral twist-boat cyclohexane rings can be fused to obtain remarkably symmetric yet asymmetric [6.6]chiralane, point group T (not Th or Td), viewed down a C3-axis[7] and a C2-axis.[8] There is no nomenclature for the central carbon's chirality, though it possesses no point, plane, or higher improper axis of symmetry.
It is important to keep in mind that molecules have considerable flexibility and thus, depending on the medium, may adopt a variety of different conformations. These various conformations are themselves almost always chiral. When assessing chirality, a time-averaged structure is considered and for routine compounds, one should refer to the most symmetric possible conformation.
When the optical rotation for an enantiomer is too low for practical measurement, it is said to exhibit cryptochirality.
Even isotopic differences must be considered when examining chirality. Replacing one of the two 1H atoms at the CH2 position of benzyl alcohol with a deuterium (¬≤H) makes that carbon a stereocenter. The resulting benzyl-őĪ-d alcohol exists as two distinct enantiomers, which can be assigned by the usual stereochemical naming conventions. The S enantiomer has [őĪ]D = +0.715¬į.[9]
Normally when an atom has four different substituents, it is chiral. However in rare cases, two of the ligands differ from each other by being mirror images of each other. When this happens, the mirror image of the molecule is identical to the original, and the molecule is achiral. This is called psuedochirality.
Properties of enantiomers
Normally, enantiomers behave identically. For example, they will migrate identically Rfs in TLC. Their NMR IR spectra are identical. Differences between optical isomers arise in the presence of other chiral molecules or objects. For example, enantiomers do not migrate identically on chiral chromatographic media, such as quartz. NMR spectra are sensitive to chiral additives (see Eufod).
Chiral compounds rotate plane polarized light. Each enantiomer will rotate the light in a different sense, clockwise or counterclockwise. Molecules that do this are said to be optically active.
Chacteristically, different enantiomers of chiral compounds often taste and smell differently and have different effects as drugs (see below). These effects reflect the chirality inherent in biological systems.
One chiral 'object' that interacts differently with the two enantiomers of a chiral compound is circularly polarized light: An enantiomer will absorb left- and right-circularly polarized light to differing degrees. This is the basis of circular dichroism (CD) spectroscopy. Usually the difference in absorptivity is relatively small (parts per thousand). CD spectroscopy is a powerful analytical technique for investigating the secondary structure of proteins and for determining the absolute configurations of chiral compounds, in particular, transition metal complexes. CD spectroscopy is replacing polarimetry as a method for characterizing chiral compounds, although the latter is still popular with sugar chemists.
In biology
Many biologically active molecules are chiral, including the naturally occurring amino acids (the building blocks of proteins), and sugars. In biological systems, most of these compounds are of the same chirality: most amino acids are L and sugars are D. Typical naturally occurring proteins, made of L amino acids, are known as left-handed proteins, whereas D amino acids produce right-handed proteins.
The origin of this homochirality in biology is the subject of much debate.[10] Most scientists believe that Earth life's "choice" of chirality was purely random, and that if carbon-based life forms exist elsewhere in the universe, their chemistry could theoretically have opposite chirality.
Enzymes, which are chiral, often distinguish between the two enantiomers of a chiral substrate. Imagine an enzyme as having a glove-like cavity that binds a substrate. If this glove is right-handed, then one enantiomer will fit inside and be bound, whereas the other enantiomer will have a poor fit and is unlikely to bind.
D-form amino acids tend to taste sweet, whereas L-forms are usually tasteless. Spearmint leaves and caraway seeds, respectively, contain L-carvone and D-carvone - enantiomers of carvone. These smell different to most people because our olfactory receptors also contain chiral molecules that behave differently in the presence of different enantiomers.
Chirality is important in context of ordered phases as well, for example the addition of a small amount of an optically active molecule to a nematic phase (a phase that has long range orientational order of molecules) transforms that phase to a chiral nematic phase (or cholesteric phase). Chirality in context of such phases in polymeric fluids has also been studied in this context.[11]
In drugs
Many chiral drugs must be made with high enantiomeric purity due to potential side-effects of the other enantiomer. (Alternatively, the other enantiomer may be merely inactive.)
- Thalidomide: Thalidomide is racemic. One enantiomer is effective against morning sickness, whereas the other is teratogenic. In this case, administering just one of the enantiomers to a pregnant patient does not help, as the two enantiomers are readily interconverted in vivo. Thus, if a person is given either enantiomer, both the D and L isomers will eventually be present in the patient's serum.
- Ethambutol: Whereas one enantiomer is used to treat tuberculosis, the other causes blindness.
- Naproxen: One enantiomer is used to treat arthritis pain, but the other causes liver poisoning with no analgesic effect.
- Steroid receptor sites also show stereoisomer specificity.
- Penicillin's activity is stereodependent. The antibiotic must mimic the D-alanine chains that occur in the cell walls of bacteria in order to react with and subsequently inhibit bacterial transpeptidase enzyme.
- Only L-propranolol is a powerful adrenoceptor antagonist, whereas D-propranolol is not. However, both have local anesthetic effect.
- The L-isomer of Methorphan, levomethorphan is a potent opioid analgesic, while the D-isomer, dextromethorphan is a dissociative cough suppressant.
- S(-) isomer of carvedilol, a drug that interacts with adrenoceptors, is 100 times more potent as beta receptor blocker than R(+) isomer. However, both the isomers are approximately equipotent as alpha receptor blockers.
- The D-isomers of amphetamine and methamphetamine are strong CNS stimulants, while the L-isomers of both drugs lack appreciable CNS(central nervous system) stimulant effects, but instead stimulate the peripheral nervous system. For this reason, the Levo-isomer of methamphetamine is available as an OTC nasal inhaler in some countries, while the Dextro-isomer is banned from medical use in all but a few countries in the world, and highly regulated in those countries who do allow it to be used medically.
In inorganic chemistry
- Main article: Complex Chemistry
Many coordination compounds are chiral; for example, the well-known [Ru(2,2'-bipyridine)3]2+ complex in which the three bipyridine ligands adopt a chiral propeller-like arrangement.[12] In this case, the Ru atom may be regarded as a stereogenic center, with the complex having point chirality. The two enantiomers of complexes such as [Ru(2,2'-bipyridine)3]2+ may be designated as őõ (left-handed twist of the propeller described by the ligands) and őĒ (right-handed twist). Hexol is a chiral cobalt complex that was first investigated by Alfred Werner. Resolved hexol is significant as being the first compound devoid of carbon to display optical activity.
Chirality of amines
Tertiary amines (see image) are chiral in a way similar to carbon compounds: The nitrogen atom bears four distinct substituents counting the lone pair. However, the energy barrier for the inversion of the stereocenter is, in general, about 30 kJ/mol, which means that the two stereoisomers are rapidly interconverted at room temperature. As a result, amines such as NHRR' cannot be resolved optically and NRR'R" can only be resolved when the R, R', and R" groups are constrained in cyclic structures.
Theories about origin of chiral compounds
A paper published in February 29, 2008 by researchers led by Sandra Pizzarello, from Arizona State University, reveals that the Murchison meteorite contains sizable molecular asymmetry of up to 14 percent, "giving support to the idea that biomolecular traits such as chiral asymmetry could have been seeded in abiotic chemistry ahead of life."[13] "Thanks to the pristine nature of this meteorite, we were able to demonstrate that other extraterrestrial amino acids carry the left-handed excesses in meteorites and, above all, that these excesses appear to signify that their precursor molecules, the aldehydes, also carried such excesses," Pizzarello said. "In other words, a molecular trait that defines life seems to have broader distribution as well as a long cosmic lineage."[14]
Alternative theories of the origin of chirality on Earth have also been proposed, such as the weak nuclear force.
| ||||||||||||||
See also
- Stereochemistry
- Axial chirality
Notes
- ‚ÜĎ A. Lakhtakia, (ed.). 1990, "Selected Papers on Natural Optical Activity." SPIE Milestone. 15.
- ‚ÜĎ L. Pasteur, 1848 (1905). "Researches on the molecular asymmetry of natural organic products." English translation of French original. Alembic Club Reprints. 14: 1-46.
- ‚ÜĎ J.C. Bose, 1898. "On the rotation of plane of polarization of electric waves by a twisted structure." Proc. R. Soc. Lond. 63: 146-152.
- ‚ÜĎ Ernest L. Eliel and Samuel H. Wilen. 1994. Stereochemistry of organic compounds. New York, NY: Wiley-Interscience. ISBN 9780471016700.
- ‚ÜĎ Pedro Cintas, 2007, "Tracing the Origins and Evolution of Chirality and Handedness in Chemical Language." Angewandte Chemie, International Edition. 46(22): 4016-4024.
- ‚ÜĎ Infelicitous stereochemical nomenclatures for stereochemical nomenclature. uottawa.ca. Retrieved February 20, 2009.
- ‚ÜĎ S-Chiralane, C3-axis. mazepath.com.
- ‚ÜĎ S-Chiralane, C2-axis. mazepath.com
- ‚ÜĎ A. Streitwieser, Jr., J.R. Wolfe, Jr., and W.D. Schaeffer. 1959. "Stereochemistry of the Primary Carbon. X. Stereochemical Configurations of Some Optically Active Deuterium Compounds." Tetrahedron. 6: 338‚Äď344.
- ‚ÜĎ Uwe J. Meierhenrich, 2008, Amino Acids and the Asymmetry of Life. (Berlin, DE: Springer. ISBN 9783540768852).
- ‚ÜĎ M. Srinivasarao, 1999, "Chirality and Polymers." Current Opinion in Colloid and Interface Science. 4(5): 369-376.
- ‚ÜĎ Alex von Zelewsky, 1996, Stereochemistry of Coordination Compounds. (Chichester, UK; New York, NY: Wiley. ISBN 9780471955993).
- ‚ÜĎ Sandra Pizzarello, Yongsong Huang, and Marcelo R. Alexandre, 2008, Molecular asymmetry in extraterrestrial chemistry: Insights from a pristine meteorite. Proceedings of the National Academy of Sciences. Retrieved February 20, 2009.
- ‚ÜĎ Arizona State University, 2008, Key To Life Before Its Origin On Earth May Have Been Discovered. ScienceDaily. Retrieved February 20, 2009.
ReferencesISBN links support NWE through referral fees
- Anslyn, Eric V., and Dennis A. Dougherty. 2004. Modern Physical Organic Chemistry. Sausalito, CA: University Science. ISBN 9781891389313.
- Eliel, Ernest Ludwig, Samuel H. Wilen, and Lewis N. Mander. 1994. Stereochemistry of Organic Compounds. New York, NY: Wiley. ISBN 0471016705.
- March, Jerry. 1985. Advanced Organic Chemistry: Reactions, Mechanisms and Structure. 3rd ed. New York, NY: John Wiley & Sons. ISBN 0471854727.
- Meierhenrich, Uwe J. 2008. Amino Acids and the Asymmetry of Life. Berlin, DE: Springer. ISBN 9783540768852.
- Mislow, Kurt Martin. 2002. Introduction to Stereochemistry. Mineola, NY: Dover Publications. ISBN 0486425304.
- Morris, David G. 2002. Stereochemistry, Basic Concepts in Chemistry. New York, NY: Wiley-Interscience. ISBN 0471224774.
- von Zelewsky, Alex. 1996. Stereochemistry of Coordination Compounds. Chichester, UK; New York, NY: Wiley. ISBN 9780471955993.
External links
All links retrieved December 10, 2023.
- Stereoisomerism - Optical ismerism.
- IUPAC nomenclature for amino acid configurations.
- Chirality & Odour Perception at leffingwell.com.
- Chirality & Bioactivity I.: Pharmacology.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.