Lysine
| Lysine | |
|---|---|
|
|
| |
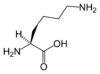
| IUPAC name | 2,6-diaminohexanoic acid |
| Other names | Lys, K |
| Identifiers | |
| CAS number | [] |
| PubChem | |
| EINECS number | |
| MeSH | |
| SMILES | C(CCN)CC(C(=O)O)N |
| Properties | |
| Molecular formula | C6H14N2O2 |
| Molar mass | 146.188 |
| Melting point |
224 °C |
| Acidity (pKa) | 2.15, 9.16, 10.67 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Lysine is an α-amino acid that is present in many proteins, has low available concentration in certain popular agricultural crops, such as wheat, and has important dietary implications. The L-isomer of lysine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Lysine also is classified as an "essential amino acid" since it cannot be synthesized by the human body from other compounds through chemical reactions and thus has to be taken in with the diet.
Lysine is low in concentration in the proteins of many cereal grains and vegetables or this amino acid is not fully biologically available. Diets poor in lysine, such as ones based on grains, can cause lysine deficiency, which will slow down protein synthesis and result in the body not being able to sustain growth and repair of muscle tissue (Longe 2005). Lysine is also important for producing antibodies, enzymes, and hormones (Longe 2005).
For those practicing vegetarian or low-fat diets, or whose staple food are grains, it is especially important to exercise discipline in eating habits to make sure that one's diet has proper amounts of this limiting amino acid. Human creativity has developed means to synthesize lysine commercially and it is often a supplement to bread, rice, and cereal-based animal feeds (Bender and Bender 2005). Efforts are underway to create crops, such as maize (corn), that are rich in lysine.
Lysine's three letter code is Lys, its one letter code is K, its codons are AAA and AAG, and its systematic name is 2,6-diaminohexanoic acid.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R | H2N-C-COOH | H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In lysine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
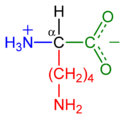
Lysine's chemical formula is NH2-(CH2)4- CH(NH2)-COOH, or in general form C6H14N2O2 (IUPAC-IUB 1983).
Lysine is a basic amino acid, as are arginine and histidine. Lysine behaves similarly to arginine. It contains a long flexible side-chain with a positively-charged end. The flexibility of the chain makes lysine and arginine suitable for binding to molecules with many negative charges on their surfaces; for example, DNA-binding proteins have their active regions rich with arginine and lysine. The strong charge makes these two amino acids prone to be located on the outer hydrophilic surfaces of the proteins.
The ε-amino group often participates in hydrogen bonding and as a general base in catalysis. Common posttranslational modifications include methylation of the e-amino group, giving methyl-, dimethyl-, and trimethyllysine. The latter occurs in calmodulin. Other posttranslational modifications include acetylation. Collagen contains hydroxylysine, which is derived from lysine by lysyl hydroxylase. O-Glycosylation of lysine residues in the endoplasmic reticulum or Golgi apparatus is used to mark certain proteins for secretion from the cell.
Sources
As an essential amino acid, lysine is not synthesized in animals, hence it must be ingested as lysine or lysine-containing proteins. The human nutritional requirement is 1–1.5 g daily.
Lysine is the limiting amino acid in many cereals, such as wheat, and thus lysine deficiency can be a problem in certain vegetarian and low-fat diets (Bender and Bender 2005; Longe 2005). Furthermore, not all the lysine in protein is biologically available, since some is linked to sugars or other amino acids through its side-chain amino group and these linkages are not hydrolyzed by digestive enzymes (Bender and Bender 2005). Lysine can be obtained from various meats (chicken, cattle, turkey) and particular vegetables.
Food rich in lysine includes milk, soybeans, meat, lentils, and spinach (Longe 2005). Fish also is quite rich in lysine. While low in all cereal grains, lysine is plentiful in all pulses (legumes). Other plants that contain significant amounts of lysine include buffalo gourd, berro, watercress, soybean, and common bean (black bean, dwarf bean, green bean, kidney bean, navy bean, string bean, etc.).
Lysine is often used as a dietary supplement.
Biosynthesis
In plants and microorganisms, lysine is synthesized from aspartic acid, which is first converted to β-aspartyl-semialdehyde. Cyclization gives dihydropicolinate, which is reduced to Δ1-piperidine-2,6-dicarboxylate. Ring-opening of this heterocycle gives a series of derivatives of pimelic acid, ultimately affording lysine. Enzymes involves in this biosynthesis include (Lehninger 2000):
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- dihydropicolinate synthase
- Δ1-piperdine-2,6-dicarboxylate dehydrogenase
- N-succinyl-2-amino-6ketopimelate synthase
- succinyl diaminopimelate aminotransferase
- succinyl diaminopimelate desuccinylase
- diaminopimelate epimerase
- diaminopimelate decarboxylase
In terms of commerical produciton, synthetic, racemic lysine (equal portions of l- and d-lysine) has long been known (Braun 1909). A practical synthesis starts from caprolactam (Eck and Marvel 1943).
Lysine is metabolised in mammals to give acetyl-CoA, via an initial transamination with α-ketoglutarate. The bacterial degradation of lysine yields cadaverine by decarboxylation.
Function
L-Lysine is a necessary building block for all protein in the body. It has noted roles in building muscle protein, tissue repair and growth, and the body's production of hormones, enzymes, and antibodies (Longe 2005).
L-Lysine plays a major role in calcium absorption (helping to prevent osteoporosis), and slows eye damage caused by diabetes (Longe 2005). It is important in recovering from surgery or sports injuries.
It has been suggested that lysine may be beneficial for those with herpes simplex infections (Griffith et al. 1978). Longe (2005) states that lysine suppresses the growth of the herpes virus, whereas arginine increase growth of this virus, and thus supplements of L-lysine are given to increase the ratio of lysine to arginine in the body, curing the outbreak of the virus. It likewise is advised to avoid foods high in arginine (geletin, nuts, chocolate) and eat foods with high lysine content in order to alleviate the symptoms of the virus (cold sores, canker sores, genital sores) (Longe 2005). However, more research is needed to fully substantiate this claim.
ReferencesISBN links support NWE through referral fees
- Much of the information in this article has been translated from German Wikipedia.
- Bender, D. A., and A. E. Bender. 2005. A Dictionary of Food and Nutrition. New York: Oxford University Press. ISBN 0198609612.
- Braun, J. V. 1909. Synthese des inaktiven Lysins aus Piperidin. Berichte der deutschen chemischen Gesellschaft 42:839-846.
- Eck, J. C., and C. S. Marvel. 1943. dl-Lysine Hydrochlorides Organic Syntheses 2: 374. Retrieved January 5, 2008.
- Griffith, R. S., A. L. Norins, and C. Kagan. 1978. A multicentered study of lysine therapy in Herpes simplex infection. Dermatologica 156(5): 257-267. PMID 640102
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB. Retrieved January 5, 2008.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
- Longe, J. L. 2005. The Gale Encyclopedia of Alternative Medicine. Detroit: Thomson Gale. ISBN 0787674249.
External links
All links retrieved March 27, 2025.
- Lysine biosynthesis (early stages), Lysine biosynthesis (later stages), and Lysine catabolism at Queen Mary, University of London.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.