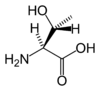
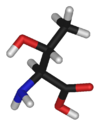
Threonine
| Threonine | |
|---|---|
| Systematic name | (2S,3R)-2-Amino- 3-hydroxybutanoic acid |
| Abbreviations | Thr T |
| Chemical formula | C4H9NO3 |
| Molecular mass | 119.12 g mol-1 |
| Melting point | 256 °C |
| Density | ? g cm-3 |
| Isoelectric point | 5.60 |
| pKa | 2.20 8.96 |
| PubChem | 6288 |
| CAS number | [72-19-5] |
| EINECS number | 200-774-1 |
| SMILES | C[C@@H](O)[C@H](N)C(O)=O |
| |
| Disclaimer and references | |
Threonine is an α-amino acid that is common in many proteins and together with serine and tyrosine is one of three proteinogenic amino acids bearing an alcohol group. Like serine, threonine is sometimes in substantial concentrations in the outer regions of soluble proteins due to its hydrophilic nature. With an easily removed hydrogen on the hydroxyl side chain, threonine is often a hydrogen donor in enzymes.
The L-isomer of threonine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Threonine is also classified as an "essential amino acid" since it cannot be synthesized by the human body from other compounds through chemical reactions and thus has to be taken in with the diet.
Beyond its role as a key building block of proteins, the role of threonine in human metabolism is unclear. However, its role in proteins is vital. For proteins to fold and function correctly, the amino acid constituents require a particular arrangement, reflecting the complex coordination in nature. Threonine also reflects an element of human responsibility, for one's diet must contain sufficient threonine to synthesize the proteins. In general, the occurrence of "essential amino acids" requires that human beings interact with and depend upon a wide diversity of other organisms, plants and animals, in order to receive their nutritional requirements.
Threonine's three letter code is Thr, its one letter code is T, its codons are ACU and ACA, and its systematic name is 2-Amino-3-hydroxybutanoic acid (IUPAC-IUB 1983).
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acidsâthose amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called αâcarbon (alpha carbon). The general structure of these alpha amino acids is:
R | H2N-C-COOH | H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis.
With two chiral centers, threonine can exist in four possible stereoisomers, or two possible diastereomers (not mirror images) of L-threonine. However, the name L-threonine is used for one single enantiomer, (2S, 3R)-2-amino-3-hydroxybutanoic acid. This is the only form used in mammalian proteins. The second diastereomer (2S, 3S), which is rarely present in nature, is called L-allo-threonine. Stereoisomers are molecules whose atomic connectivity is the same but whose atomic arrangement in space is different. Enantiomers are stereoisomers that are nonsuperposable complete mirror images of each other, much as one's left and right hands are "the same" but opposite.
Threonine has the chemical formula CH3-CH(OH)-CH(NH2)-COOH, or more generally, C4H9NO3.
Threonine, like serine, has a short group ended with a hydroxyl group. The hydroxyl group attached makes it a polar amino acid. Its hydrogen is easy to remove, so threonine and serine often act as hydrogen donors in enzymes. However, while serine has a reputation as being involved in catalytic functions in enzymes, such as in trypsin and chymotrypsin, threonine's role is this respect is not settled. Both threonine and serine are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them.
The threonine residue (component) is susceptible to numerous posttranslational modifications. The hydroxy side chain can undergo O-linked glycosylation (addition of saccharides). Additionally, threonine residues undergo phosphorylation (addition of phosphate) through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine.
Source
As an essential amino acid, threonine is not synthesized in humans, hence we must ingest threonine or, more commonly, threonine-containing proteins. Fortunately, most proteins contain threonine and so a deficiency is unlikely. Foods high in threonine include milk, cottage cheese, poultry, fish, meat, lentils, sesame seeds, eggs, beans, corn, and various grains.
Biosynthesis
In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group (Lehninger 2000). Enzymes involved in a typical biosynthesis of threonine include:
- aspartokinase
- α-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine kinase
- threonine synthase
Function, metabolism, and synthesis
Other than an essential constituent of proteins, threonine's role in metabolism in mammals and humans has not been delineated. It is used in biochemical and nutritional research. It is also given as a dietary supplement. In bacteria, threonine is involved in the biosynthesis of vitamin B12 (cobalamin) and the amino acid isoleucine.
Threonine is metabolized in two ways:
- It is converted to pyruvate
- It is converted to alpha-ketobutyrate, and thereby enters the pathway leading to succinyl CoA.
Racemic threonine (equal portions of L and D threonine) can be prepared in the laboratory from crotonic acid by alpha-functionalization using mercury(II) acetate (Carter and West 1955).
ReferencesISBN links support NWE through referral fees
- Carter, H. E., and H. D. West. âdl-threonine.â Organic Syntheses, 3: 813, 1955.
- Doolittle, R. F. âRedundancies in protein sequences.â In G. D. Fasman, ed. Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press, 1989. ISBN 0306431319
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB, 1983. Retrieved September 25, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing, 2000. ISBN 1572591536
External links
All links retrieved April 30, 2023.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.