Methionine
| Methionine | |
|---|---|
| Systematic name | (S)-2-amino-4-(methylsulfanyl)- butanoic acid |
| Abbreviations | Met M |
| Chemical formula | C5H11NO2S |
| Molecular mass | 149.21 g mol-1 |
| Melting point | 281 °C |
| Density | 1.340 g cm-3 |
| Isoelectric point | 5.74 |
| pKa | 2.16 9.08 |
| CAS number | [63-68-3] |
| PubChem | 876 |
| EINECS number | 200-562-9 |
| SMILES | CSCC[C@H](N)C(O)=O |
| |
| Disclaimer and references | |
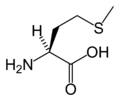
Methionine is an α-amino acid present in many proteins and, together with cysteine, is one of two sulfur-containing proteinogenic amino acids. Methionine is the source of sulfur for many compounds, including cysteine (Longe 2005). The derivative S-adenosyl methionine (SAM) is an important methyl donor involved in a variety of biochemical pathways and in the synthesis of epinephrine, choline, and other substances.
The L-isomer of methionine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Methionine also is classified as an "essential amino acid" since it cannot be synthesized by the human body from other compounds through chemical reactions and thus has to be taken in with the diet.
Methionine is one of only two amino acids encoded by a single codon (AUG) in the standard genetic code. (Tryptophan, encoded by UGG, is the other). The codon AUG is also significant in that it carries the "Start" message for a ribosome to begin protein translation from mRNA. As a consequence, methionine is incorporated into the N-terminal position of all proteins in eukaryotes and archaea during translation, although it is usually removed by post-translational modification. This role of methionine reveals the remarkable unity among living organisms; furthermore, the precision required for a functional protein, involving a particular arrangement of amino acids and specific three-dimensional folding of the protein, reflects on the complex coordination in nature.
Methionine's three letter code is Met, its one letter code is M, and its systematic name is 2-Amino-4-(methylthio)butanoic acid.
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R | H2N-C-COOH | H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In methionine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Methionine's chemical formula is CH3-S-(CH2)2)-CH(NH2)-COOH, or in general form C5H11NO2S (IUPAC-IUB 1983).
Like cysteine, methionine contains sulfur, but with a methyl group instead of hydrogen. This methyl group (one carbon and three hydrogen, or CH3) can be activated, and is used in many reactions where a new carbon atom is being added to another molecule. Methionine is classified as nonpolar. Methionine is always the first amino acid to be incorporated into a protein; it is sometimes removed after translation.
Sources
As an essential amino acid, methionine is not synthesized in animals, hence it must be ingested as methionine or methionine-containing proteins. High levels of methionine can be found in sesame seeds, Brazil nuts, fish, meat, and some seeds. Most fruit and vegetables contain very little, although peppers and spinach are the best sources. Legumes are not a good source.
Biosynthesis
In plants and microorganisms, methionine is synthesized via a pathway that uses both of the amino acids aspartic acid and cysteine. First, aspartic acid is converted via β-aspartyl-semialdehyde into homoserine, introducing the pair of contiguous methylene groups. Homoserine converts to O-succinyl homoserine, which then reacts with cysteine to produce cystathionine, which is cleaved to yield homocysteine. Subsequent methylation of the thiol group by folates affords methionine.
Both cystathionine-γ-synthase and cystathionine-β-lyase require Pyridoxyl-5'-phosphate as a cofactor, whereas homocysteine methyltransferase requires Vitamin B12 as a cofactor (Lehninger 2000).
Enzymes involved in methionine biosynthesis:
- aspartokinase
- β-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine acyltransferase
- cystathionine-γ-synthase
- cystathionine-β-lyase
- methionine synthase (in mammals, this step is performed by homocysteine methyltransferase)
Other biochemical pathways
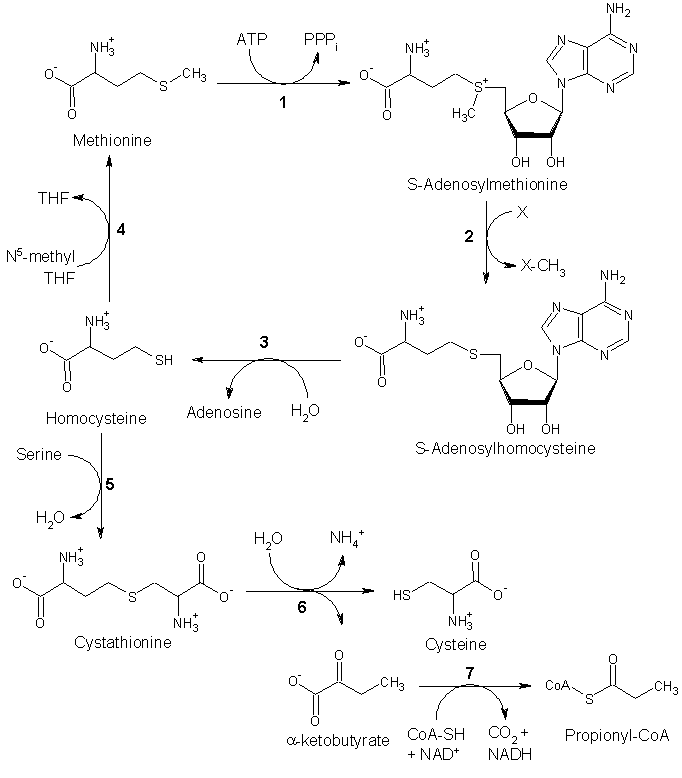
S-adenosyl-l-methionine (SAM or SAMe) is a compound that is formed from a reaction of methionine and adenosine triphosphate (ATP). SAM is the main methyl donor in the human body, involved in a variety of biochemical pathways.
Methionine is converted to SAM by (1) methionine adenosyltransferase. SAM then serves as a methyl donor in many (2) methyltransferase reactions and is converted to S-adenosylhomocysteine (SAH). (3) Adenosylhomocysteinase converts SAH to homocysteine.
There are two fates of homocysteine.
- First, methionine can be regenerated from homocysteine via (4) methionine synthase. It can also be remethylated using glycine betaine (NNN-trimethyl glycine) to methionine via the enzyme Betaine-homocysteine methyltransferase (E.C.2.1.1.5, BHMT). BHMT makes up to 1.5 percent of all the soluble protein of the liver, and recent evidence suggests that it may have a greater influence on methionine and homocysteine homeostasis than methionine synthase.
- Alternatively, homocysteine can be converted to cysteine. (5) Cystathionine-β-synthase (a PLP-dependent enzyme) combines homocysteine and serine to produce cystathionine. Instead of degrading cystathionine via cystathionine-β-lyase as in the biosynthetic pathway, cystathionine is broken down to cysteine and α-ketobutyrate via (6) cystathionine-γ-lyase. (7) α-ketoacid dehydrogenase converts α-ketobutyrate to propionyl-CoA, which is metabolized to succinyl-CoA in a three-step process.
Synthesis
Racemic methionine (equal portions of L- and D-sterioisomers) can be synthesized from diethyl sodium phthalimidomalonate, (C6H4(CO)2NC(CO2Et)2), by alkylation with chloroethylmethylsulfide, ClCH2CH2SCH3 followed by hydrolysis and decarboxylation (Barger and Weichselvaum 1943).
Function
Methionine and its derivatives fulfill many important functions in the body.
- Source of sulfur: Methionine is an important sources of sulfur for numerous compounds, such as the amino acids cysteine and taurine (Longe 2005). Sulfur is used by the body for health hair, skin and nail growth, to increase the livers production of lecithin, reduction of liver fat, protection of the kidneys, in the excretion of heavy metals, and regulating the formation of ammonia in the urine (Longe 2005).
- S-adenosyl-l-methionine (SAM) reactions: SAM, a derivative of methionine, is involved in the synthesis of epinephrine , choline, and other substances, and is an important methyl donor (see above).
- Lipotropic: Methionine is a lipotropic, helping to prevent fat accumulation in the liver and typically aiding the detoxification of metabolic wastes and toxins (Longe 2005).
- Acetaminophen overdose treatment: Methionine is used in the treatment of acetaminophen poisoning to prevent liver damage (Longe 2005).
- Arthritis treatment: SAM is used to provide relief from arthritis and is said to have fewer side effects than common anti-inflammatory drugs like ibuprofen and aspirin (Longe 2005).
- Depression: SAM is prescribed more than any of antidepressant and is considered as effective, quick acting, and with fewer side effects, and may boost the activity of key brain chemicals involved in mood, such as norepinephrine, dopamine, and serotonin (Longe 2005).
- Liver function: Methionine levels are used in determining the liver’s concentration of sulfur-containing compounds and SAM is used in improving and normalizing liver function (Longe 2005). It is used in treatment of cirrhosis and alcohol damage and is key to production of glutathione (Longe 2005). It also inactivates estrogens to prevent suppressed bile flow in pregnant women or those on oral contraceptives (Longe 2005).
- Neurological disorders: SAM is considered to improve binding of neurotranmsitters to receptor sites in the brain and the regeneration of neuron axons following injury.
Methionine also plays a role in the synthesis of phosphatidylcholine, and other phospholipids. Improper conversion of methionine can lead to atherosclerosis.
ReferencesISBN links support NWE through referral fees
- Barger, G., and T. E. Weichselbaum. 1943. dl-methionine Organic Syntheses 2: 384. Retrieved September 27, 2016.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology IUPAC-IUB. Retrieved September 27, 2016
- Longe, J. L., ed. 2005. The Gale Encyclopedia of Alternative Medicine. Detroit: Thomson/Gale.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.