Aluminum
| |||||||||||||||||||||||||
| General | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | aluminum, Al, 13 | ||||||||||||||||||||||||
| Chemical series | poor metals | ||||||||||||||||||||||||
| Group, Period, Block | 13, 3, p | ||||||||||||||||||||||||
| Appearance | silvery
| ||||||||||||||||||||||||
| Standard atomic weight | 26.9815386(8) g·mol−1 | ||||||||||||||||||||||||
| Electron configuration | [Ne] 3s2 3p1 | ||||||||||||||||||||||||
| Electrons per shell | 2, 8, 3 | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||
| Density (near r.t.) | 2.70 g·cm−3 | ||||||||||||||||||||||||
| Liquid density at m.p. | 2.375 g·cm−3 | ||||||||||||||||||||||||
| Melting point | 933.47 K (660.32 °C, 1220.58 °F) | ||||||||||||||||||||||||
| Boiling point | 2792 K (2519 °C, 4566 °F) | ||||||||||||||||||||||||
| Heat of fusion | 10.71 kJ·mol−1 | ||||||||||||||||||||||||
| Heat of vaporization | 294.0 kJ·mol−1 | ||||||||||||||||||||||||
| Heat capacity | (25 °C) 24.200 J·mol−1·K−1 | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Crystal structure | face centered cubic 0.4032 nm | ||||||||||||||||||||||||
| Oxidation states | 3 (amphoteric oxide) | ||||||||||||||||||||||||
| Electronegativity | 1.61 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies (more) |
1st: 577.5 kJ·mol−1 | ||||||||||||||||||||||||
| 2nd: 1816.7 kJ·mol−1 | |||||||||||||||||||||||||
| 3rd: 2744.8 kJ·mol−1 | |||||||||||||||||||||||||
| Atomic radius | 125 pm | ||||||||||||||||||||||||
| Atomic radius (calc.) | 118 pm | ||||||||||||||||||||||||
| Covalent radius | 118 pm | ||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||
| Electrical resistivity | (20 °C) 26.50 nΩ·m | ||||||||||||||||||||||||
| Thermal conductivity | (300 K) 237 W·m−1·K−1 | ||||||||||||||||||||||||
| Thermal expansion | (25 °C) 23.1 µm·m−1·K−1 | ||||||||||||||||||||||||
| Speed of sound (thin rod) | (r.t.) (rolled) 5000 m·s−1 | ||||||||||||||||||||||||
| Young's modulus | 70 GPa | ||||||||||||||||||||||||
| Shear modulus | 26 GPa | ||||||||||||||||||||||||
| Bulk modulus | 76 GPa | ||||||||||||||||||||||||
| Poisson ratio | 0.35 | ||||||||||||||||||||||||
| Mohs hardness | 2.75 | ||||||||||||||||||||||||
| Vickers hardness | 167 MPa | ||||||||||||||||||||||||
| Brinell hardness | 245 MPa | ||||||||||||||||||||||||
| CAS registry number | 7429-90-5 | ||||||||||||||||||||||||
| Selected isotopes | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
- "Aluminium" redirects here.
Aluminum (or aluminium) (chemical symbol Al, atomic number is 13) is a soft, lightweight metal with a silvery appearance and the ability to resist corrosion. It is the most abundant metallic element in the Earth's crust (estimated at between 7.5 and 8.1 percent). The free element, rarely found in nature, occurs in oxygen-deficient environments such as volcanic mud. Its main ore is bauxite. Whether measured in terms of quantity or value, the global use of aluminum exceeds that of any other metal except iron, and it is important in virtually all segments of the world economy.
Structural components made from aluminum and its alloys are vital to the aerospace industry and very important in other areas of transportation and building. In addition, aluminum and its alloys are used in packaging, cooking utensils, electrical transmission lines, water purification processes, electronic devices and compact discs, paint, and pyrotechnics. Aluminum compounds also serve a wide variety of purposes. For instance, aluminum ammonium sulfate is a mordant for dyeing, and is used in water purification and sewage treatment; aluminum acetate solution is an astringent; aluminum chloride is used in paints and anti-perspirants; and aluminum borate, phosphate, and fluorosilicate are used in the production of glass and ceramics. Yet, aluminum is one of the few abundant elements that appear to have no beneficial biological role; a small percentage of people are allergic to it.
History
Ancient Greeks and Romans used aluminum salts as mordants for dyeing and astringents for dressing wounds. Alum (potassium aluminum sulfate or a related salt) is still used as a styptic. In 1761, Guyton de Morveau suggested calling the base alum alumine. In 1808, Humphry Davy identified the existence of a metal base of alum, which he at first named alumium and later aluminum (see Spelling section, below).
Friedrich Wöhler is generally credited with isolating aluminum (Latin alumen, alum) in 1827 by mixing anhydrous aluminum chloride with potassium. The metal, however, had been produced (albeit in impure form) for the first time two years earlier by the Danish physicist and chemist Hans Christian Ørsted. Therefore, Ørsted can also be listed as the discoverer of the metal.[1] Further, Pierre Berthier discovered aluminum in bauxite ore and successfully extracted it.[2] The Frenchman Henri Etienne Sainte-Claire Deville improved Wöhler's method in 1846 and described his improvements in a book in 1859, chief among these being the substitution of sodium for the considerably more expensive potassium.[3]
Before the development of methods to purify aluminum in large quantities, it was considered a precious metal more valuable than gold. Napoleon III, Emperor of France, is reputed to have given a banquet where the most honored guests were given aluminum utensils, while the other guests had to make do with gold ones.[4][5]
Aluminum was selected as the material to be used for the apex of the Washington Monument in 1884, a time when a single ounce (30 grams) of the substance cost the daily wage of a common worker on the project.[6] It had about the same value as silver.
In 1886, the American Charles Martin Hall of Oberlin, Ohio applied for a patent (U.S. patent 400664 ) for an electrolytic process to extract aluminum using the same technique that was independently being developed by the Frenchman Paul Héroult in Europe. The invention of the Hall-Héroult process in 1886 made extracting aluminum from minerals cheaper, and it is now the principal method used throughout the world. The Hall-Heroult process, however, cannot produce Super Purity Aluminum directly. Upon approval of his patent in 1889, Hall, with the financial backing of Alfred E. Hunt of Pittsburgh, PA, started the Pittsburgh Reduction Company, which was renamed the Aluminum Company of America in 1907 and later shortened to Alcoa.
Germany became the world leader in aluminum production soon after Adolf Hitler's rise to power. By 1942, however, new hydroelectric power projects such as the Grand Coulee Dam gave the United States something Nazi Germany could not compete with, provided them with sufficient generating capacity to produce enough aluminum to manufacture sixty thousand warplanes in four years.
Notable characteristics
Physical properties
In the periodic table, aluminum is located in group 13 (former group 3A), between boron and gallium. In addition, it lies in period 3, between magnesium and silicon. It is considered a member of the "poor metal" group of chemical elements.[7] It is nontoxic, nonmagnetic, and nonsparking. The atoms in the metal are arranged in a face-centered cubic structure.
Aluminum is one of the few metals that retain full silvery reflectance in finely powdered form, making it an important component of silver paints. Pure aluminum serves as an excellent reflector (approximately 99%) of visible light and a good reflector (approximately 95%) of infrared. It is a good thermal and electrical conductor, by weight better than copper. It is capable of being a superconductor, with a superconducting critical temperature of 1.2 Kelvin.
This metal has about one-third the density and stiffness of steel. It is ductile, and easily machined, cast, and extruded. The yield strength of pure aluminum is 7-11 MPa, while aluminum alloys have yield strengths ranging from 200 to 600 MPa.[8] Also, pure aluminum has a low tensile strength, but its alloys display a marked improvement in mechanical properties, especially when tempered.
Chemical properties
Aluminum is highly resistant to corrosion, due to a thin surface layer of aluminum oxide that forms when the metal is exposed to air, effectively preventing further oxidation. The strongest aluminum alloys are less corrosion resistant due to galvanic reactions with alloyed copper.[9]
When combining with other elements, aluminum can have different oxidation states: +1, +2, and +3. Of these, the +3 oxidation state is most common.
Oxidation state one:[10]
- AlH is produced when aluminum is heated at 1500 °C in an atmosphere of hydrogen.
- Al2O is made by heating the normal oxide, Al2O3, with silicon at 1800 °C in a vacuum.
- Al2S can be made by heating Al2S3 with aluminum shavings at 1300 °C in a vacuum. It quickly breaks up to regenerate the starting materials. The selenide is made in a parallel manner.
- AlF, AlCl, and AlBr exist in the gaseous phase when the corresponding tri-halide is heated with aluminum.
Oxidation state two:
- Aluminum monoxide, AlO, is present when aluminum powder burns in oxygen.
Oxidation state three:
- According to Fajans' rules, the simple trivalent cation Al3+ is not expected to be found in anhydrous salts or binary compounds such as Al2O3. The hydroxide is a weak base and aluminum salts of weak acids, such as carbonate, can't be prepared. The salts of strong acids, such as nitrate, are stable and soluble in water, forming hydrates with at least six molecules of water of crystallization.
- Aluminum hydride, (AlH3)n, can be produced from trimethylaluminum and an excess of hydrogen. It burns explosively in air. It can also be prepared by the action of aluminum chloride on lithium hydride in ether solution, but cannot be isolated free from the solvent.
- Aluminum carbide, Al4C3 is made by heating a mixture of the elements above 1000 °C. The pale yellow crystals have a complex lattice structure, and react with water or dilute acids to give methane. The acetylide, Al2(C2)3, is made by passing acetylene over heated aluminum.
- Aluminum nitride, AlN, can be made from the elements at 800 °C. It is hydrolyzed by water to form ammonia and aluminum hydroxide.
- Aluminum phosphide, AlP, is made similarly, and hydrolyses to give phosphine.
- Aluminum oxide, Al2O3, occurs naturally as corundum, and can be made by burning aluminum in oxygen or by heating the hydroxide, nitrate or sulfate. As a gemstone, its hardness is only exceeded by diamond, boron nitride, and carborundum. It is almost insoluble in water.
- Aluminum hydroxide may be prepared as a gelatinous precipitate by adding ammonia to an aqueous solution of an aluminum salt. It is amphoteric, being both a very weak acid and forming aluminates with alkalis. It exists in various crystalline forms.
- Aluminum sulfide, Al2S3, may be prepared by passing hydrogen sulfide over aluminum powder. It is polymorphic.
- Aluminum iodide, (AlI3)2, is a dimer with applications in organic synthesis.
- Aluminum fluoride, AlF3, is made by treating the hydroxide with HF, or can be made from the elements. It consists of a giant molecule which sublimes without melting at 1291 °C. It is very inert. The other trihalides are dimeric, having a bridge-like structure.
- Aluminum fluoride/water complexes: When aluminum and fluoride are together in aqueous solution, they readily form complex ions such as AlF(H2O)5+2, AlF3(H2O)30, AlF6-3. Of these, AlF6-3 is the most stable. This is explained by the fact that aluminum and fluoride, which are both very compact ions, fit together just right to form the octahedral aluminum hexafluoride complex. When aluminum and fluoride are together in water in a 1:6 molar ratio, AlF6-3 is the most common form, even in rather low concentrations.
- Organo-metallic compounds of empirical formula AlR3 exist and, if not also giant molecules, are at least dimers or trimers. They have some uses in organic synthesis, for instance trimethylaluminum.
- Alumino-hydrides of the most electropositive elements are known, the most useful being lithium aluminum hydride, Li[AlH4]. It decomposes into lithium hydride, aluminum and hydrogen when heated, and is hydrolysed by water. It has many uses in organic chemistry, particularly as a reducing agent. The aluminohalides have a similar structure.
Clusters
In the journal Science of January 14, 2005, it was reported that clusters of 13 aluminum atoms (Al13) had been made to behave like an iodine atom; and, 14 aluminum atoms (Al14) behaved like an alkaline earth atom. The researchers also bound 12 iodine atoms to an Al13 cluster to form a new class of polyiodide. This discovery is reported to give rise to the possibility of a new characterization of the periodic table: superatoms. The research teams were led by Shiv N. Khanna (Virginia Commonwealth University) and A. Welford Castleman, Jr. (Penn State University).[11]
Isotopes
Aluminum has many isotopes, of which only 27Al (stable isotope) and 26Al (radioactive isotope, t1/2 = 7.2 × 105 y) occur naturally. The 27Al isotope has a natural abundance of 99.9+ percent. 26Al is produced from argon in the atmosphere by spallation caused by cosmic-ray protons. Aluminum isotopes have found practical application in dating marine sediments, manganese nodules, glacial ice, quartz in rock exposures, and meteorites. The ratio of 26Al to 10Be has been used to study the role of transport, deposition, sediment storage, burial times, and erosion on 105 to 106 year time scales.
Cosmogenic 26Al was first applied in studies of the Moon and meteorites. Meteorite fragments, after departure from their parent bodies, are exposed to intense cosmic-ray bombardment during their travel through space, causing substantial 26Al production. After falling to Earth, atmospheric shielding protects the meteorite fragments from further 26Al production, and its decay can then be used to determine the meteorite's terrestrial age. Meteorite research has also shown that 26Al was relatively abundant at the time of formation of our planetary system. Many researchers studying meteorites believe that the energy released by the decay of 26Al was responsible for the melting and differentiation of some asteroids after their formation 4.55 billion years ago.[12]
Aluminum metal production and refinement
Aluminum is a reactive metal that is difficult to extract from ore, aluminum oxide (Al2O3). Direct reduction—with carbon, for example—is not economically viable since aluminum oxide has a melting point of about 2,000 °C. Therefore, it is extracted by electrolysis; that is, the aluminum oxide is dissolved in molten cryolite and then reduced to the pure metal. By this process, the operational temperature of the reduction cells is around 950 to 980 °C. Cryolite is found as a mineral in Greenland, but in industrial use it has been replaced by a synthetic substance. Cryolite is a mixture of aluminum, sodium, and calcium fluorides: (Na3AlF6). The aluminum oxide (a white powder) is obtained by refining bauxite in the Bayer process. (Previously, the Deville process was the predominant refining technology.)
The electrolytic process replaced the Wöhler process, which involved the reduction of anhydrous aluminum chloride with potassium. Both of the electrodes used in the electrolysis of aluminum oxide are carbon. Once the ore is in the molten state, its ions are free to move around. The reaction at the cathode (the negative terminal) produces aluminum metal:
- Al3+ + 3 e− → Al
Here, the aluminum ion is reduced (electrons are added). The aluminum metal then sinks to the bottom and is tapped off.
At the positive electrode (anode), oxygen is formed:
- 2 O2− → O2 + 4 e−
This carbon anode is then oxidized by the oxygen, releasing carbon dioxide. The anodes in a reduction cell must therefore be replaced regularly, since they are consumed in the process:
- O2 + C → CO2
Unlike the anodes, the cathodes are not oxidized because there is no oxygen present at the cathode. The carbon cathode is protected by the liquid aluminum inside the cells. Nevertheless, cathodes do erode, mainly due to electrochemical processes. After five to ten years, depending on the current used in the electrolysis, a cell has to be rebuilt because of cathode wear.
Aluminum electrolysis with the Hall-Héroult process consumes a lot of energy, but alternative processes were always found to be less viable economically and/or ecologically. The world-wide average specific energy consumption is approximately 15±0.5 kilowatt-hours per kilogram of aluminum produced from alumina. (52 to 56 MJ/kg). The most modern smelters reach approximately 12.8 kW·h/kg (46.1 MJ/kg). Reduction line current for older technologies are typically 100 to 200 kA. State-of-the-art smelters operate with about 350 kA. Trials have been reported with 500 kA cells.
Recovery of the metal via recycling has become an important facet of the aluminum industry. Recycling involves melting the scrap, a process that uses only five percent of the energy needed to produce aluminum from ore. However, a significant part (up to 15% of input material) is lost as dross (ash-like oxide). Recycling was a low-profile activity until the late 1960s, when the growing use of aluminum beverage cans brought it to the public consciousness.
Electric power represents about 20 to 40 percent of the cost of producing aluminum, depending on the location of the smelter. Smelters tend to be situated where electric power is both plentiful and inexpensive, such as South Africa, the South Island of New Zealand, Australia, the People's Republic of China, the Middle East, Russia, Quebec and British Columbia in Canada, and Iceland.
Over the last 50 years, Australia has become a major producer of bauxite ore and a major producer and exporter of alumina.[13] Australia produced 62 million metric tons of bauxite in 2005. The Australian deposits have some refining problems, some being high in silica but have the advantage of being shallow and relatively easy to mine.[14]
Applications
General uses
Relatively pure aluminum is prepared only when corrosion resistance or workability is more important than strength or hardness. This metal readily forms alloys with many elements such as copper, zinc, magnesium, manganese, and silicon. Aluminum alloys form vital components of aircraft and rockets as a result of their high strength-to-weight ratio. Today, almost all bulk metal materials that are referred to loosely as "aluminum," are actually alloys. For example, the common aluminum foils are alloys containing 92-99% aluminum.[15]
Some of the many uses for aluminum metal are in:
- Transportation (particularly automobiles, aircraft, trucks, railroad cars, marine vessels, and bicycles)
- Packaging (such as cans and foil)
- Optical coatings and mirrors, in which a thin layer of aluminum is deposited on a flat surface.
- Water treatment
- Treatment against fish parasites such as Gyrodactylus salaris
- Construction (windows, doors, siding, building wire, etc.)
- Cooking utensils
- Electrical transmission lines for power distribution
- MKM steel and Alnico magnets
- Super purity aluminum (SPA, 99.980 percent to 99.999 percent Al), used in electronics and CDs.
- Heat sinks for electronic appliances such as transistors and CPUs.
- Powdered aluminum is used in paint, and in pyrotechnics such as solid rocket fuels and thermite.
- The blades of prop swords and knives used in stage combat.
Aluminum compounds
- Aluminum ammonium sulfate ([Al(NH4)](SO4)2), ammonium alum is used as a mordant, in water purification and sewage treatment, in paper production, as a food additive, and in leather tanning.
- Aluminum acetate is a salt used in solution as an astringent.
- Aluminum borohydride (Al(BH4)3) is used as an additive to jet fuel.
- Aluminum chloride (AlCl3) is used: in paint manufacturing, in antiperspirants, in petroleum refining and in the production of synthetic rubber.
- Aluminum chlorohydride is used as an antiperspirant and in the treatment of hyperhidrosis.
- Aluminum fluorosilicate (Al2(SiF6)3) is used in the production of synthetic gemstones, glass and ceramic.
- Aluminum hydroxide (Al(OH)3) is used: as an antacid, as a mordant, in water purification, in the manufacture of glass and ceramic and in the waterproofing of fabrics.
- Aluminum oxide (Al2O3), alumina, is found naturally as corundum (rubies and sapphires), emery, and is used in glass making. Synthetic ruby and sapphire are used in lasers for the production of coherent light.
- Aluminum phosphate (AlPO4) is used in the manufacture: of glass and ceramic, pulp and paper products, cosmetics, paints and varnishes and in making dental cement.
- Aluminum sulfate (Al2(SO4)3) is used: in the manufacture of paper, as a mordant, in a fire extinguisher, in water purification and sewage treatment, as a food additive, in fireproofing, and in leather tanning.
- In many vaccines, certain aluminum salts serve as an immune adjuvant (immune response booster) to allow the protein in the vaccine to achieve sufficient potency as an immune stimulant.
Aluminum alloys in structural applications
Aluminum alloys with a wide range of properties are used in engineering structures. Alloy systems are classified by a number system (ANSI) or by names indicating their main alloying constituents (DIN and ISO).
Aluminum is used extensively in many places due to its high strength to weight ratio. However, a designer used to working with steel will find aluminum less well-behaved in terms of flexibility. The problems may often be addressed by redesigning parts dimensionally specifically to address issues of stiffness.
The strength and durability of aluminum alloys varies widely, not only as a result of the components of the specific alloy, but also as a result of heat treatments and manufacturing processes. A lack of knowledge of these aspects has from time to time led to improperly designed structures and given aluminum a bad reputation.
One important structural limitation of aluminum alloys is their fatigue strength. Unlike steels, aluminum alloys have no well defined fatigue limit, meaning that fatigue failure will eventually occur under even very small cyclic loadings. This implies that engineers must assess these loads and design for a fixed life rather than an infinite life.
Another important property of aluminum alloys is their sensitivity to heat. Workshop procedures involving heating are complicated by the fact that aluminum, unlike steel, will melt without first glowing red. Forming operations where a blow torch is used therefore requires some expertise, since no visual signs reveal how close the material is to melting. Aluminum alloys, like all structural alloys, also are subject to internal stresses following heating operations such as welding and casting. The problem with aluminum alloys in this regard is their low melting point, which make them more susceptible to distortions from thermally induced stress relief. Controlled stress relief can be done during manufacturing by heat-treating the parts in an oven, followed by gradual cooling - in effect annealing the stresses.
The low melting point of aluminum alloys has not precluded their use in rocketry; even for use in constructing combustion chambers where gases can reach 3500 K. The Agena upper stage engine used a regeneratively cooled aluminum design for some parts of the nozzle, including the thermally critical throat region; in fact the extremely high thermal conductivity of aluminum prevented the throat from reaching the melting point even under massive heat flux, resulting in a reliable and lightweight component.
Household wiring
Aluminum has about 65 percent of the conductivity of copper, the traditional household wiring material. In the 1960s aluminum was considerably cheaper than copper, and so was introduced for household electrical wiring in the United States, even though many fixtures had not been designed to accept aluminum wire. However, in some cases the greater coefficient of thermal expansion of aluminum causes the wire to expand and contract relative to the dissimilar metal screw connection, eventually loosening the connection. Also, pure aluminum has a tendency to "creep" under steady sustained pressure (to a greater degree as the temperature rises), again loosening the connection. Finally, Galvanic corrosion from the dissimilar metals increased the electrical resistance of the connection.
All of this resulted in overheated and loose connections, and this in turn resulted in some fires. Builders then became wary of using the wire, and many jurisdictions outlawed its use in very small sizes, in new construction. Eventually, newer fixtures were introduced with connections designed to avoid loosening and overheating. At first they were marked "Al/Cu," but they now bear a "CO/ALR" coding. In older assemblies, workers forestall the heating problem using a properly done crimp of the aluminum wire to a short "pigtail" of copper wire. Today, new alloys, designs, and methods are used for aluminum wiring in combination with aluminum terminations.
Precautions
Aluminum is a neurotoxin that alters the function of the blood-brain barrier.[16] It is one of the few abundant elements that appears to have no beneficial function in living cells. A small percent of people are allergic to it — they experience contact dermatitis from any form of it: an itchy rash from using styptic or antiperspirant products, digestive disorders, an inability to absorb nutrients from eating food cooked in aluminum pans, and vomiting and other symptoms of poisoning from ingesting such products as Amphojel, and Maalox (antacids). In other people, aluminum is not considered as toxic as heavy metals, but there is evidence of some toxicity if it is consumed in excessive amounts. The use of aluminum cookware, popular because of its corrosion resistance and good heat conduction, has not been shown to lead to aluminum toxicity in general. Excessive consumption of antacids containing aluminum compounds and excessive use of aluminum-containing antiperspirants are more likely causes of toxicity. In research published in the Journal of Applied Toxicology, Dr. Philippa D. Darby of the University of Reading has shown that aluminum salts increase estrogen-related gene expression in human breast cancer cells grown in the laboratory. These salts' estrogen-like effects have lead to their classification as metalloestrogens.
It has been suggested that aluminum is a cause of Alzheimer's disease, as some brain plaques have been found to contain the metal. Research in this area has been inconclusive; aluminum accumulation may be a consequence of the Alzheimer's damage, not the cause. In any event, if there is any toxicity of aluminum it must be via a very specific mechanism, since total human exposure to the element in the form of naturally occurring clay in soil and dust is enormously large over a lifetime.[17][18]
Mercury applied to the surface of an aluminum alloy can damage the protective oxide surface film by forming amalgam. This may cause further corrosion and weakening of the structure. For this reason, mercury thermometers are not allowed on many airliners, as aluminum is used in many aircraft structures.
Powdered aluminum can react with Fe2O3 to form Fe and Al2O3. This mixture is known as thermite, which burns with a high energy output. Thermite can be produced inadvertently during grinding operations, but the high ignition temperature makes incidents unlikely in most workshop environments.
Aluminum and plants
Aluminum is primary among the factors that contribute to the loss of plant production on acid soils. Although it is generally harmless to plant growth in pH-neutral soils, the concentration in acid soils of toxic Al3+ cations increases and disturbs root growth and function.
The adaptation of wheat to allow aluminum tolerance is such that the aluminum induces a release of organic compounds that bind to the harmful aluminum cations. Sorghum is believed to have the same tolerance mechanism. The first gene for aluminum tolerance has been identified in wheat. A group in the U.S. Department of Agriculture showed that sorghum's aluminum tolerance is controlled by a single gene, as for wheat. This is not the case in all plants.
Spelling
Etymology/nomenclature history
The earliest citation given in the Oxford English Dictionary for any word used as a name for this element is alumium, which Humphry Davy employed in 1808 for the metal he was trying to isolate electrolytically from the mineral alumina. The citation is from his journal Philosophical Transactions: "Had I been so fortunate as.. to have procured the metallic substances I was in search of, I should have proposed for them the names of silicium, alumium, zirconium, and glucium."[19]
By 1812, Davy had settled on aluminum, which (as other sources note) matches its Latin root. He wrote in the journal Chemical Philosophy: "As yet Aluminum has not been obtained in a perfectly free state."[20] But the same year, an anonymous contributor to the Quarterly Review, a British political-literary journal, objected to aluminum and proposed the name aluminium, "for so we shall take the liberty of writing the word, in preference to aluminum, which has a less classical sound."[21]
The -ium suffix had the advantage of conforming to the precedent set in other newly discovered elements of the time: potassium, sodium, magnesium, calcium, and strontium (all of which Davy had isolated himself). Nevertheless, -um spellings for elements were not unknown at the time, as for example platinum, known to Europeans since the sixteenth century, molybdenum, discovered in 1778, and tantalum, discovered in 1802.
Americans adopted -ium for most of the nineteenth century, with aluminium appearing in Webster's Dictionary of 1828. In 1892, however, Charles Martin Hall used the -um spelling in an advertising handbill for his new electrolytic method of producing the metal, despite his constant use of the -ium spelling in all the patents he filed between 1886 and 1903.[22] It has consequently been suggested that the spelling reflects an easier to pronounce word with one fewer syllable, or that the spelling on the flier was a spelling mistake. Hall's domination of production of the metal ensured that the spelling aluminum became the standard in North America; the Webster Unabridged Dictionary of 1913, though, continued to use the -ium version.
In 1926, the American Chemical Society officially decided to use aluminum in its publications; American dictionaries typically label the spelling aluminium as a British variant.
Present-day spelling
In the UK and other countries using British spelling, only aluminium is used. In the United States, the spelling aluminium is largely unknown, and the spelling aluminum predominates.[23][24] The Canadian Oxford Dictionary prefers aluminum, whereas the Australian Macquarie Dictionary prefers aluminium.
In other English-speaking countries, the spellings (and associated pronunciations) aluminium and aluminum are both in common use in scientific and nonscientific contexts. The spelling in virtually all other languages is analogous to the -ium ending.
The International Union of Pure and Applied Chemistry (IUPAC) adopted aluminium as the standard international name for the element in 1990, but three years later recognized aluminum as an acceptable variant. Hence their periodic table includes both, but places aluminium first.[25] IUPAC officially prefers the use of aluminium in its internal publications, although several IUPAC publications use the spelling aluminum.
See also
Notes
- ↑ Yinon Bentor. Periodic Table: Aluminum ChemicalElements.com. Retrieved August 13, 2007.
- ↑ Pierre Berthier. Today in Science History. Retrieved August 13, 2007.
- ↑ The title of Deville's book is De l'aluminium, ses propriétés, sa fabrication (Paris, 1859). It is likely that Deville also thought of the idea of the electrolysis of aluminum oxide dissolved in cryolite. However, Charles Martin Hall and Paul Heroult might have developed the more practical process after Deville.
- ↑ S. Venetski, 1969. "Silver" from clay. Metallurgist 13(7): 451-453.
- ↑ ChemMatters magazine. (1990): 14
- ↑ George J. Binczewski, 1995. The Point of a Monument: A History of the Aluminum Cap of the Washington Monument. Retrieved August 13, 2007.
- ↑ The term poor metals (or post-transition metals) refers to the metallic elements in the p-block of the periodic table. Their melting and boiling points are generally lower than those of the transition metals and their electronegativity higher, and they are also softer. In addition to aluminum, the group includes gallium, indium, thallium, tin, lead, and bismuth.
- ↑ Polmear, I. J. 1995. Light Alloys. London, UK: Arnold Publishers. ISBN 0750663715
- ↑ Ibid.
- ↑ The temperatures in this section seem to be the subject of controversy.
- ↑ Clusters of Aluminum Atoms Found to Have Properties of Other Elements Reveal a New Form of Chemistry. Eberly College of Science. Retrieved August 13, 2007.
- ↑ Robert T. Dodd, 1986. Thunderstones and Shooting Stars. (Cambridge, MA: Harvard University Press.), 89-90.
- ↑ The Australian Industry. Australian Aluminium Council. Retrieved August 13, 2007.
- ↑ Australian Bauxite. Australian Aluminium Council. Retrieved August 13, 2007.
- ↑ L. S. Millberg, Aluminum Foil. How Products are Made. Retrieved August 13, 2007.
- ↑ W.A. Banks and A.J. Kastin. 1989. Aluminum-induced neurotoxicity: alterations in membrane function at the blood-brain barrier. Neurosci Biobehav Rev 13(1):47-53.
- ↑ Alzheimer's Disease and Aluminum. National Institute of Environmental Health Sciences.
- ↑ Michael Hopkin, 2006. Death of Alzheimer victim linked to aluminium pollution. news @ nature.com.
- ↑ "alumium," Oxford English Dictionary, 2nd ed. Edited by J.A. Simpson and E.S.C. Weiner. (Oxford, UK: Clarendon Press, 1989). OED Online Oxford University Press. Accessed October 29, 2006. Citation is listed as "1808 SIR H. DAVY in Phil. Trans. XCVIII. 353." The ellipsis in the quotation is as it appears in the OED citation.
- ↑ "aluminum," Ibid. Citation is listed as "1812 SIR H. DAVY Chem. Philos. I. 355"
- ↑ "aluminium," Ibid. Citation is listed as "1812 Q. Rev. VIII. 72"
- ↑ Peter Meiers. Manufacture of Aluminum. The History of Fluorine, Fluoride and Fluoridation. Retrieved August 13, 2007.
- ↑ Greenwood, N. N.; & Earnshaw, A. 1997. Chemistry of the Elements (2nd Edn.). Oxford, UK: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ↑ John Bremner. 1980. Words on Words: A Dictionary for Writers and Others Who Care about Words. (New York, NY: Columbia University Press), 22–23.
- ↑ IUPAC Periodic Table of the Elements. Retrieved August 13, 2007.
ReferencesISBN links support NWE through referral fees
- Bremner, John. 1980. Words on Words: A Dictionary for Writers and Others Who Care about Words. New York, NY: Columbia University Press. ISBN 0231044933
- Chang, Raymond. 2006. Chemistry, 9th ed. New York, NY: McGraw-Hill Science/Engineering/Math. ISBN 0073221031.
- Cotton, F. Albert, and Geoffrey Wilkinson. 1980. Advanced Inorganic Chemistry, 4th ed. New York, NY: Wiley. ISBN 0471027758.
- Dodd, Robert T. 1986. Thunderstones and Shooting Stars. Cambridge, MA: Harvard University Press. ISBN 0674891376.
- Greenwood, N.N., and A. Earnshaw. 1998. Chemistry of the Elements, 2nd ed. Oxford, U.K.; Burlington, MA: Butterworth-Heinemann, Elsevier Science. ISBN 0750633654. Online version available here. Retrieved August 11, 2007.
- Los Alamos National Laboratory Periodic Table Aluminum. Chemistry Division, Los Alamos National Laboratory. Retrieved August 11, 2007.
- Polmear, I. J. 1995. Light Alloys. London, UK: Arnold Publishers. ISBN 0750663715
External links
All links retrieved July 23, 2023.
- Aluminium WebElements.com.
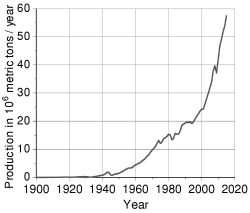
- World production of primary aluminium, by country.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.