Carbon
Carbon is a remarkable chemical element for many reasons. It is a vital component of all known living systems, and without it life as we know it could not exist (see alternative biochemistry). Its different forms include graphite, one of the softest known substances, and diamond, one of the hardest substances. Carbon is known to be part of a huge variety of different compounds, including some that occur in the Sun, stars, and planetary atmospheres. Many carbon compounds are of economic importance. We use them for fuel and the synthesis of a wide range of new materials such as plastics, paints, textiles, and pharmaceuticals.
On the other hand, compounds of carbon can pose environmental and health hazards, if not used carefully. For instance, the careless disposal of paints and plastics and the misuse of drugs can be dangerous for humans and other living organisms. We therefore need to be wise in terms of our production, usage, and disposal of carbon compounds.
| |||||||||||||||||||||||||
| General | |||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | carbon, C, 6 | ||||||||||||||||||||||||
| Chemical series | nonmetals | ||||||||||||||||||||||||
| Group, Period, Block | 14, 2, p | ||||||||||||||||||||||||
| Appearance | black (graphite) colorless (diamond) 
| ||||||||||||||||||||||||
| Atomic mass | 12.0107(8) g/mol | ||||||||||||||||||||||||
| Electron configuration | 1s2 2s2 2p2 | ||||||||||||||||||||||||
| Electrons per shell | 2, 4 | ||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||
| Density (near r.t.) | (graphite) 2.267 g/cm³ | ||||||||||||||||||||||||
| Density (near r.t.) | (diamond) 3.513 g/cm³ | ||||||||||||||||||||||||
| Melting point | ? triple point, ca. 10 MPa and (4300–4700) K (? °C, ? °F) | ||||||||||||||||||||||||
| Boiling point | subl. ? ca. 4000 K (? °C, ? °F) | ||||||||||||||||||||||||
| Heat of fusion | (graphite) ? 100 kJ/mol | ||||||||||||||||||||||||
| Heat of fusion | (diamond) ? 120 kJ/mol | ||||||||||||||||||||||||
| Heat of vaporization | ? 355.8 kJ/mol | ||||||||||||||||||||||||
| Heat capacity | (25 °C) (graphite) 8.517 J/(mol·K) | ||||||||||||||||||||||||
| Heat capacity | (25 °C) (diamond) 6.115 J/(mol·K) | ||||||||||||||||||||||||
| |||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||
| Crystal structure | hexagonal | ||||||||||||||||||||||||
| Oxidation states | 4, 2 (mildly acidic oxide) | ||||||||||||||||||||||||
| Electronegativity | 2.55 (Pauling scale) | ||||||||||||||||||||||||
| Ionization energies (more) |
1st: 1086.5 kJ/mol | ||||||||||||||||||||||||
| 2nd: 2352.6 kJ/mol | |||||||||||||||||||||||||
| 3rd: 4620.5 kJ/mol | |||||||||||||||||||||||||
| Atomic radius | 70 pm | ||||||||||||||||||||||||
| Atomic radius (calc.) | 67 pm | ||||||||||||||||||||||||
| Covalent radius | 77 pm | ||||||||||||||||||||||||
| Van der Waals radius | 170 pm | ||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||
| Magnetic ordering | diamagnetic | ||||||||||||||||||||||||
| Thermal conductivity | (300 K) (graphite) (119–165) W/(m·K) | ||||||||||||||||||||||||
| Thermal conductivity | (300 K) (diamond) (900–2320) W/(m·K) | ||||||||||||||||||||||||
| Thermal diffusivity | (300 K) (diamond) (503–1300) mm²/s | ||||||||||||||||||||||||
| Mohs hardness | (graphite) 0.5 | ||||||||||||||||||||||||
| Mohs hardness | (diamond) 10.0 | ||||||||||||||||||||||||
| CAS registry number | 7440-44-0 | ||||||||||||||||||||||||
| Notable isotopes | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
Occurrence
Carbon is an abundant nonmetal that has been known since prehistory. Early peoples produced it in the form of charcoal by burning organic material (such as wood) in insufficient oxygen.
The name carbon comes from the French word charbone, which in turn is derived from the Latin carbo, meaning charcoal. In German and Dutch, the names for carbon are Kohlenstoff and koolstof, respectively, both literally meaning "coal-stuff".
Pure carbon can occur in a variety of forms known as allotropes, such as graphite and diamond, described below. In addition, carbon can bind to itself and various other elements to form compounds. Scientists are aware of nearly 10 million carbon compounds, constituting the vast majority of all known chemical compounds. Most compounds of carbon are classified as organic compounds, which form the basis for the field of organic chemistry.
Thousands of carbon compounds—including carbohydrates, fats, proteins, and nucleic acids—are produced in living systems. Other compounds, particularly the carbonates of metals (including calcium, magnesium, and iron), are major components of rocks such as limestone, dolomite, and marble. In addition, a number of carbon compounds, classified as hydrocarbons, occur in coal, petroleum, and natural gas.
In the United States, graphite is found in large quantities in New York and Texas. It is also abundant in Russia, Mexico, Greenland, and India.
Natural diamonds occur in the mineral kimberlite found in ancient volcanic "necks" or "pipes." Most diamond deposits are in Africa, notably in South Africa, Namibia, Botswana, the Republic of the Congo and Sierra Leone. Other deposits have been found in Arkansas, Canada, the Russian Arctic, Brazil, and Northern and Western Australia.
Additional allotropic forms of carbon, classified as fullerenes, were discovered as byproducts of molecular beam experiments in the 1980s.
Carbon is abundant in the Sun and stars, and it is thought that carbon atoms were first formed by nuclear reactions in the interior of stars. Carbon is also found in comets and in the atmospheres of most planets in the solar system. Some meteorites contain microscopic diamonds that may have been formed when the solar system was in its formative stages.
Properties of carbon
The chemical symbol for carbon is C, and its atomic number (the number of protons in the nucleus of each atom) is 6.
Each carbon atom is capable of forming strong chemical bonds, called covalent bonds, with up to four other atoms. Carbon is therefore said to be tetravalent. When bound to oxygen, it forms carbon dioxide and carbon monoxide. When combined with hydrogen, it forms various hydrocarbons, including methane and propane. When attached to both oxygen and hydrogen, it forms many groups of compounds, including alcohols, esters, carbohydrates, and fatty acids. Other organic compounds include proteins and nucleic acids.
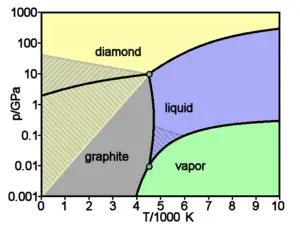
Carbon has the highest melting/sublimation point of all elements. At atmospheric pressure, it has no actual melting point as its triple point is at 10 MPa (100 bar) so it sublimates above 4000 K. Thus it remains solid at higher temperatures than the highest melting point metals like tungsten or rhenium, regardless of its allotropic form.
Scientists believe that carbon was first created not during the Big Bang but in the interior of stars. It is thought that each carbon atom was produced by the collision of three alpha particles (helium nuclei).
Applications
Carbon is a vital component of all known living systems, and without it life as we know it could not exist (see alternative biochemistry). The major economic use of carbon is in the form of hydrocarbons, most notably the fossil fuels methane gas and crude oil (petroleum). Crude oil is used by the petrochemical industry to produce, amongst others, gasoline and kerosene, through a distillation process in refineries. Crude oil forms the raw material for many synthetic substances, many of which are collectively called plastics.
Other uses
- The isotope Carbon-14 was discovered in February 27 1940 and is used in radiocarbon dating.
- Graphite is combined with clays to form the 'lead' used in pencils.
- Diamond is used for decorative purposes, and also as drill bits and other applications making use of its hardness. Diamonds have long been considered rare and beautiful.
- Carbon is added to iron to make steel.
- Carbon is used as a neutron moderator in nuclear reactors.
- Graphite carbon in a powdered, caked form is used as charcoal for cooking, artwork and other uses.
- Activated charcoal is used in medicine (as powder or compounded in tablets or capsules) to absorb toxins or poisons from the digestive system.
The chemical and structural properties of fullerenes, in the form of carbon nanotubes, has promising potential uses in the nascent field of nanotechnology. Nanoparticles might however be toxic.
Allotropes
carbon has several allotropic forms:
- Diamond (hardest known natural mineral). Structure: each atom is bonded tetrahedrally to four others, making a 3-dimensional network of puckered six-membered rings of atoms.
- Graphite (one of the softest substances). Structure: each atom is bonded trigonally to three other atoms, making a 2-dimensional network of flat six-membered rings; the flat sheets are loosely bonded.
- Fullerenes. Structure: comparatively large molecules formed completely of carbon bonded trigonally, forming spheroids (of which the best-known and simplest is the buckminsterfullerene or buckyball).
- Chaoite A mineral supposedly formed in meteorite impacts.
- Lonsdaleite (a corruption of diamond). Structure: similar to diamond, but forming a hexagonal crystal lattice.
- Amorphous carbon (a glassy substance). Structure: an assortment of carbon molecules in a non-crystalline, irregular, glassy state.
- Carbon nanofoam (an extremely light magnetic web). Structure: a low-density web of graphite-like clusters, in which the atoms are bonded trigonally in six- and seven-membered rings.
- Carbon nanotubes (tiny tubes). Structure: each atom is bonded trigonally in a curved sheet that forms a hollow cylinder.
- Aggregated diamond nanorods, the most recently discovered allotrope and the hardest substance known to man.
Lamp black consists of small graphitic areas. These areas are randomly distributed, so the whole structure is isotropic.
'Glassy carbon' is isotropic and contains a high proportion of closed porosity. Unlike normal graphite, the graphitic layers are not stacked like pages in a book, but have a more random arrangement.
Carbon fibers are similar to glassy carbon. Under special treatment (stretching of organic fibers and carbonization) it is possible to arrange the carbon planes in direction of the fiber. Perpendicular to the fiber axis there is no orientation of the carbon planes. The result is fibers with a higher specific strength than steel.
Pure carbon occurs in different forms known as allotropes. These forms are based on different molecular configurations.
The three relatively well-known allotropes of carbon are amorphous carbon, graphite, and diamond. In addition, several exotic allotropes have been synthesized or discovered, including fullerenes, carbon nanotubes, lonsdaleite and aggregated diamond nanorods.
In its amorphous form, carbon is essentially graphite but not held in a crystalline macrostructure. It is, rather, present as a powder which is the main constituent of substances such as charcoal, lamp black (soot) and activated carbon.
At normal pressures, carbon takes the form of graphite, in which each atom is bonded to three others in a plane composed of fused hexagonal rings, just like those in aromatic hydrocarbons. The two known forms of graphite, alpha (hexagonal) and beta (rhombohedral), both have identical physical properties, except for their crystal structure. Graphites that naturally occur have been found to contain up to 30% of the beta form, when synthetically-produced graphite only contains the alpha form. The alpha form can be converted to the beta form through mechanical treatment and the beta form reverts back to the alpha form when it is heated above 1000 °C.
Because of the delocalization of the pi-cloud, graphite conducts electricity. The material is soft and the sheets, frequently separated by other atoms, are held together only by van der Waals forces, so easily slip past one another.
At very high pressures carbon forms an allotrope called diamond, in which each atom is bonded to four others. Diamond has the same cubic structure as silicon and germanium and, thanks to the strength of the carbon-carbon bonds, is together with the isoelectronic boron nitride (BN) the hardest substance in terms of resistance to scratching. The transition to graphite at room temperature is so slow as to be unnoticeable. Under some conditions, carbon crystallizes as Lonsdaleite, a form similar to diamond but hexagonal.
Fullerenes have a graphite-like structure, but instead of purely hexagonal packing, also contain pentagons (or possibly heptagons) of carbon atoms, which bend the sheet into spheres, ellipses or cylinders. The properties of fullerenes (also called "buckyballs" and "buckytubes") have not yet been fully analyzed. All the names of fullerenes are after Buckminster Fuller, developer of the geodesic dome, which mimics the structure of "buckyballs".
A nanofoam allotrope has been discovered which is ferromagnetic.
Carbon allotropes include:
- Amorphous carbon
- Carbon nanofoam (discovered in 1997)
- Carbon nanotube
- Diamond
- Fullerene
- Graphite
- Lonsdaleite
- Aggregated diamond nanorods (synthesised in 2005)
The system of carbon allotropes spans a range of extremes.
Between diamond and graphite:
- Graphite is soft and is used in pencils
- Diamond is the hardest mineral known to man (although aggregated diamond nanorods are now believed to be even harder), but graphite is one of the softest.
- Diamond is the ultimate abrasive, but graphite is a very good lubricant.
- Diamond is an excellent electrical insulator, but graphite is a conductor of electricity.
- Diamond is usually transparent, but graphite is opaque.
- Diamond crystallizes in the cubic system but graphite crystallizes in the hexagonal system.
Between amorphous carbon and nanotubes:
- Amorphous carbon is among the easiest materials to synthesize, but carbon nanotubes are extremely expensive to make.
- Amorphous carbon is completely isotropic, but carbon nanotubes are among the most anisotropic materials ever produced.
Organic compounds
The most prominent oxide of carbon is carbon dioxide, CO2. This is a minor component of the Earth's atmosphere, produced and used by living things, and a common volatile elsewhere. In water it forms trace amounts of methanoic acid, HCO2H, but as most compounds with multiple single-bonded oxygens on a single carbon it is unstable. Through this intermediate, though, resonance-stabilized carbonate ions are produced. Some important minerals are carbonates, notably calcite. Carbon disulfide, CS2, is similar.
The other oxides are carbon monoxide, CO, and the uncommon carbon suboxide, C3O2. Carbon monoxide is formed by incomplete combustion, and is a colorless, odorless gas. The molecules each contain a triple bond and are fairly polar, resulting in a tendency to bind permanently to haemoglobin molecules, so that the gas is highly poisonous. Cyanide, CN-, has a similar structure and behaves a lot like a halide ion; the nitride cyanogen, (CN)2, is related.
With reactive metals, such as tungsten, carbon forms either carbides, C-, or acetylides, C22- to form alloys with very high melting points. These anions are also associated with methane and acetylene, both very weak acids. All in all, with an electronegativity of 2.5, carbon prefers to form covalent bonds. A few carbides are covalent lattices, like carborundum, SiC, which resembles diamond.
Carbon chains
Carbon has the ability to form long chains with interconnecting C-C bonds. This property is called Catenation. Carbon-Carbon bonds are fairly strong, and abnormaly stable. This property is important as it allows carbon to form a huge number of compounds; if fact, there are more known carbon-containing compounds than all the other compounds of the chemical elements combined!
The simplest form of an organic molecule is the hydrocarbon - a large family of organic molecules that, by definition, are composed of hydrogen atoms bonded to a chain of carbon atoms. Chain length, side chains and functional groups all affect the properties of organic molecules.
Carbon cycle
Under terrestrial conditions, conversion of one isotope to another is very rare. Therefore, for practical purposes, the amount of carbon on Earth is constant. Thus, processes that use carbon must obtain it from somewhere, and dispose of it somewhere. The paths that carbon follows in the environment are collectively called the carbon cycle. For example, plants draw carbon dioxide out of the environments and use it to build biomass. Some of this biomass is eaten by animals, where some of it is exhaled as carbon dioxide. The carbon cycle is considerably more complicated than this short loop; for example, some carbon dioxide is dissolved in the oceans; dead plant or animal matter may become sedimentary rock, and so forth.
Isotopes
Carbon has two stable, naturally-occurring isotopes: carbon-12, or 12C, (98.89%) and carbon-13, or 13C, (1.11%), and one unstable, naturally-occurring, radioisotope; carbon-14 or 14C. There are 15 known isotopes of carbon and the shortest-lived of these is 8C which decays through proton emission and alpha decay. It has a half-life of 1.98739x10-21 s.
In 1961 the International Union of Pure and Applied Chemistry adopted the isotope carbon-12 as the basis for atomic weights.
Carbon-14 has a half-life of 5730 y and has been used extensively for radioactive dating of carbonaceous materials.
Precautions
Carbon is relatively safe. Inhalation of fine soot in large quantities can be dangerous. Carbon may catch fire at very high temperatures and burn vigorously (as in the Windscale fire).
There are a tremendous number of carbon compounds; some are lethally poisonous (cyanide, CN-), and some are essential to life (dextrose).
ReferencesISBN links support NWE through referral fees
- On Graphite Transformations at High Temperature and Pressure Induced by Absorption of the LHC Beam, J.M. Zazula, 1997
- WebElements.com and EnvironmentalChemistry.com per the guidelines at Wikipedia's WikiProject Elements
See also
- Organic chemistry
- Inorganic chemistry of carbon
- Allotropes of carbon
- Diamond
- Material properties of diamond
- Carbon nanotube
External links
- Los Alamos National Laboratory – Carbon
- WebElements.com – Carbon
- It's Elemental – Carbon
- – Carbon Fullerene and other Allotropes models by Vincent Herr
- Extensive Carbon page at asu.edu
- Electrochemical uses of carbon
- Computational Chemistry Wiki
- Origin of Carbon
af:Koolstof ar:كربون bg:Въглерод bn:কার্বন ca:Carboni cs:Uhlík cy:Carbon da:Carbon de:Kohlenstoff et:Süsinik el:Άνθρακας es:Carbono eo:Karbono eu:Karbono fa:کربن fr:Carbone gd:Gualan gl:Carbono (elemento) gu:કાર્બન ko:탄소 hr:Ugljik io:Karbo id:Karbon ia:Carbon (elemento) is:Kolefni it:Carbonio he:פחמן la:Carbonium lv:Ogleklis lt:Anglis li:Koolstof hu:Szén mk:Јаглерод mi:Waro ms:Karbon nl:Koolstof nds:Kohlenstoff ja:炭素 no:Karbon (grunnstoff) nn:Karbon oc:Carbòni pl:Węgiel (pierwiastek) pt:Carbono ru:Углерод simple:Carbon sk:Uhlík sl:Ogljik sr:Угљеник su:Karbon fi:Hiili sv:Kol th:คาร์บอน tr:karbon vi:Cacbon uk:Вуглець wa:Carbone zh:碳
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.