Difference between revisions of "Water" - New World Encyclopedia
(revised 3rd para) |
Rosie Tanabe (talk | contribs) |
||
| (77 intermediate revisions by 8 users not shown) | |||
| Line 1: | Line 1: | ||
| − | '''Water | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{Copyedited}} |
| + | :''This article is about the chemical substance.'' | ||
| + | {|class="infobox" style="float:right;" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090;" | ||
| + | ! {{chembox header}} | <big>[[Water (molecule)|Water]]</big> | ||
| + | |- | ||
| + | | align="center" colspan="2" bgcolor="#ffffff" | [[Image:Water molecule dimensions.svg|135px|The dimensions and geometric structure of a water molecule]][[Image:Water molecule.svg|110px|This space-filled model shows the molecular structure of water.]] | ||
| + | Water is the base of all life, and<br/>an abundant [[chemical compound|compound]] on the Earth's surface. | ||
| + | |- | ||
| + | ! {{chembox header}} | Information and properties | ||
| + | |- | ||
| + | | [[IUPAC nomenclature|Systematic name]] | ||
| + | | water | ||
| + | |- | ||
| + | | Alternative names | ||
| + | | aqua, [[dihydrogen monoxide]], <br/>hydrogen hydroxide, ([[wikt:wikisaurus:water|more]]) | ||
| + | |- | ||
| + | | [[Molecular formula]] | ||
| + | | H<sub>2</sub>O | ||
| + | |- | ||
| + | | [[International Chemical Identifier|InChI]] | ||
| + | | InChI=1/H2O/h1H2 | ||
| + | |- | ||
| + | | [[Molar mass]] | ||
| + | | 18.0153 g/mol | ||
| + | |- | ||
| + | | [[Density]] and [[Phase (matter)|phase]] | ||
| + | | 0.998 g/cm³ <small>(liquid at 20 °C)</small><br/> 0.92 g/cm³ <small>(solid)</small> | ||
| + | |- | ||
| + | | [[Melting point]] | ||
| + | | 0 [[Celsius|°C]] (273.15 [[kelvin|K]]) (32 [[Fahrenheit|°F]]) | ||
| + | |- | ||
| + | | [[Boiling point]] | ||
| + | | 100 °C (373.15 K) (212 °F) | ||
| + | |- | ||
| + | | [[Specific heat capacity]] | ||
| + | | 4.184 J/(g·K) <small>(liquid at 20 °C)</small> | ||
| + | |- | ||
| + | |} | ||
| − | + | '''Water''' is a common [[chemical substance]] that is essential for all known forms of [[life]].<ref>Laurence D. Barron, Lutz Hecht, and Gary Wilson, [https://pubs.acs.org/doi/abs/10.1021/bi971323j The Lubricant of Life: A Proposal That Solvent Water Promotes Extremely Fast Conformational Fluctuations in Mobile Heteropolypeptide Structure] ''ACS Publications'', 1997. Retrieved January 9, 2020.</ref> In typical usage, the term ''water'' refers to its [[liquid]] [[States of matter|state]], but the substance also has a [[solid]] state, ''[[ice]],'' and a [[gaseous]] state, ''[[water vapor]].'' About 71 percent of the [[Earth]]'s surface is covered by water, mostly in oceans and other large water bodies. | |
| − | + | The presence of water on Earth depends on various factors, including the Earth's location in the [[Solar System]]. If Earth were about 5 percent closer to or farther from the [[Sun]], there would have been a much lower likelihood for the three forms of water to be present on this planet. Also, the Earth's mass is appropriate for [[gravity]] to hold an [[Earth's atmosphere|atmosphere]], in which water vapor (along with carbon dioxide) helps maintain a relatively steady surface temperature. A smaller Earth would have a thinner atmosphere, causing temperature extremes and preventing the accumulation of water except at the [[polar ice cap]]s. If Earth were much more massive, the water on it could have been in the solid state even at relatively high temperatures, because of the high pressure caused by gravity. | |
| − | + | Water moves continually through a [[water cycle|cycle]] of [[evaporation]] or [[transpiration]], [[precipitation (meteorology)|precipitation]], and [[runoff (water)|runoff]], usually reaching the [[sea]]. Winds carry water vapor over land at the same rate as runoff into the sea, about 36 Tt per year. Over land, evaporation and transpiration contribute another 71 Tt per year to the precipitation of 107 Tt per year over land. Some water is trapped for varying periods in ice caps, glaciers, aquifers, or in lakes, sometimes providing freshwater for life on land. Water is a good [[solvent]] for a wide variety of substances. | |
| − | + | Humans use water for many purposes, including drinking, cooking, cleaning, heating, and cooling. We find it valuable for scientific experimentation and industrial processes as well as for agriculture. In addition, we use water for various sports and recreational activities. In various religions, water is considered a purifier in an internal, spiritual sense as well as in an external, physical sense. Also, the [[Jordan River]], [[Ganges River]], and other bodies of water are considered sacred by people of certain religions. | |
| + | {{toc}} | ||
| + | Yet, water pollution, overconsumption, and uneven distribution have resulted in shortages of clean freshwater in many parts of the world. These shortages have in turn led to disputes between peoples of different nations. | ||
| − | + | Beyond the Earth, a significant quantity of water is thought to exist underground on the planet [[Mars]], on [[Jupiter]]'s moon [[Europa (moon)|Europa]] and [[Saturn]]'s moon [[Enceladus (moon)|Enceladus]], and also on [[exoplanet]]s such as [[HD 189733 b]]<ref> Pallab Ghosh, [https://www.bbc.com/news/science-environment-49648746 Water found for first time on 'potentially habitable' planet] ''BBC News'', September 12, 2019. Retrieved January 9, 2020.</ref> and [[HD 209458b]].<ref>Ker Than, [https://www.space.com/3673-water-extrasolar-planet-atmosphere.html Water Found in Extrasolar Planet's Atmosphere]. ''Space.com'', April 10, 2007. Retrieved January 9, 2020.</ref> | |
| − | + | [[Image:The Earth seen from Apollo 17.jpg|right|thumb|225px|Water covers about 71 percent of the [[Earth]]'s surface; the [[ocean]]s contain about 97 percent of the Earth's water. The [[Antarctic ice sheet]], which contains 90 percent of all freshwater on Earth, is visible at the bottom. Condensed atmospheric water can be seen as [[cloud]]s, contributing to the Earth's [[albedo]].]] | |
| + | [[Image:Hopetoun falls.jpg|thumb|right|250px|Hopetoun Falls near [[Otway National Park]], [[Victoria, Australia]].]] | ||
| − | [[Image: | + | == Chemical and physical properties == |
| − | + | [[Image:Trilliumlake.jpg|right|280px|thumb|Trillium Lake in the [[Mount Hood National Forest|Mt. Hood National Forest]], near Portland, Oregon.]] | |
| − | Water | + | [[Image:Water droplet blue bg05.jpg||right|thumb|280px|Impact from a water drop causes an upward "rebound" jet surrounded by circular [[capillary wave]]s.]] |
| + | Water is a [[chemical compound]] with the [[chemical formula]] '''[[hydrogen|H]]<sub>2</sub>[[oxygen|O]]'''. Each [[molecule]] of water consists of two hydrogen [[atom]]s [[covalent]]ly [[chemical bond|bonded]] to a single oxygen atom. At [[standard conditions|ambient temperature and pressure]], water is a tasteless, odorless liquid. It appears colorless in small quantities, but it has an intrinsic very light blue hue. Pure ice also appears colorless, and water vapor is essentially invisible as a gas.<ref>Charles L. Braun, Sergei N. Smirnov, Why is water blue? ''J. Chem. Educ.'' 70(8) (1993):612. </ref> | ||
| − | + | Water is primarily a liquid under standard conditions—a property that makes it different from other analogous hydrides of the [[Chalcogen|oxygen family]] in the [[periodic table]]. Those hydrides, such as [[hydrogen sulfide]], are gases. Also, the elements surrounding oxygen in the periodic table—namely, [[nitrogen]], [[fluorine]], [[phosphorus]], [[sulfur]] and [[chlorine]]—all combine with [[hydrogen]] to produce gases under standard conditions. | |
| − | + | === Polar nature of water molecules === | |
| + | Many of the properties of water can be explained by the [[polar]] nature of its molecules. The oxygen atom is strongly [[electronegative]], and within each water molecule, the oxygen atom draws [[electron]]s closer to itself, away from the [[hydrogen]] atoms. As a result, there is a partial negative charge (δ-) near the oxygen atom and a partial positive charge (δ+) near each hydrogen atom. Thus the entire molecule is polar, with a net [[dipole moment]]. Due to this [[polarity]], there is electrical attraction between water molecules, pulling them closer to one another. This attraction is called [[hydrogen bond]]ing. | ||
| − | + | The hydrogen bonds between water molecules raise the boiling point of water and cause it to be a liquid at room temperature and pressure. By contrast, [[hydrogen sulfide]] is a gas under the same conditions because of the absence of such hydrogen bonds between its molecules. | |
| − | + | === Acids, bases, and pH values === | |
| + | Water is involved in common [[acid]]-[[base (chemistry)|base]] reactions. An acid (more precisely, a Brønsted-Lowry acid) is a donor of hydrogen ions (H<sup>+</sup>, or proton), and a base (Brønsted-Lowry base) is a hydrogen ion acceptor. When the base is a hydroxide ion (OH<sup>−</sup>), its reaction (neutralization) with an acid produces water (HOH). | ||
| − | + | Some water molecules react with one another to produce [[hydronium]] ions (H<sub>3</sub>O<sup>+</sup><sub>(aq)</sub>) and [[hydroxide]] ions (OH<sup>−</sup><sub>(aq)</sub>). In this case, one water molecule acts as an acid and donates a hydrogen ion to another, which acts as a base. | |
| − | + | Water is also the usual standard for the measurement of [[pH]]—a quantity defined as the negative logarithm of the hydrogen ion concentration. When the pH of water (or a solution) is 7, it is said to be "neutral"—neither acidic nor basic. Acids (and acidic solutions) have pH values less than 7; bases (and basic solutions) have pH values greater than 7. | |
| − | + | ===Cohesion and adhesion=== | |
| + | [[Image:Water drops on spider web.jpg|thumb|250px|right|[[Dew]] drops adhering to a [[spider web]].]] | ||
| − | + | Given the polar nature of water molecules, water tends to stick to itself—a property known as [[cohesion (chemistry)|cohesion]]. At the same time, the polar nature of water molecules also explains the ability of water to stick to other surfaces—a property known as [[adhesion]]. For example, water may form a thin film on clean, smooth [[glass]] because the adhesive forces between glass and water molecules are stronger than the cohesive forces. | |
| − | + | In biological cells, water tends to stick to [[hydrophilic]] (water-attracting) surfaces of proteins and membranes. To dehydrate hydrophilic surfaces—that is, to remove the strongly held layers of water—requires doing substantial work against these forces, called hydration forces. These forces are particularly important when cells are exposed to dry atmospheres or during extracellular freezing. | |
| − | + | ===Surface tension=== | |
| + | [[Image:Dscn3156-daisy-water 1200x900.jpg|thumb|250px|right|This [[daisy]] is under the water level, which has risen gently and smoothly. Surface tension prevents the water from submerging the flower.]] | ||
| − | + | Water has a high [[surface tension]] caused by the strong cohesion between water molecules. This can be seen when small quantities of water are put onto a non-soluble surface such as [[polythene]]; the water stays together as drops. Just as significantly, air trapped in surface disturbances forms bubbles, which sometimes last long enough to transfer gas molecules to the water. | |
| − | + | Another surface tension effect is [[capillary wave]]s. These are the surface ripples that form from around the impact of drops on water surfaces, and sometimes occur when strong subsurface currents flow to the water surface. The apparent elasticity caused by surface tension drives the waves. | |
| − | + | ===Capillary action=== | |
| + | [[Capillary action]] refers to the process of water moving up a narrow tube against the force of [[gravity]]. It occurs because (a) water adheres to the sides of the tube; (b) surface tension tends to straighten the surface, making the surface rise; and (c) more water is pulled up through cohesion. The process is repeated as the water flows up the tube, until the water reaches a level where gravity counteracts the adhesive forces. | ||
| − | == | + | ===Solvation=== |
| + | [[Image:Havasu Falls 2 md.jpg|thumb|200px|right|High concentrations of dissolved [[Lime (mineral)|lime]] make the water of [[Havasu Falls]] appear turquoise.]] | ||
| − | [[ | + | Water is a very strong [[solvent]] and dissolves many types of substances. It has therefore been called the ''universal solvent.'' Substances that will mix well and dissolve in water (such as [[salt]]s) are known as "[[hydrophilic]]" (water-loving) substances; those that do not mix well with water (such as [[lipids|fats and oils]]), are called "[[hydrophobic]]" (water-fearing) substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong [[intermolecular force#Dipole-dipole interactions|attractive forces]] that water molecules generate among themselves. If the properties of a substance do not allow it to overcome these strong intermolecular forces, the molecules are "[[precipitation (chemistry)|pushed out]]" from the water and do not dissolve. |
| − | |||
| − | + | ===Electrical conductivity=== | |
| + | Pure water has low [[electrical conductivity]], but it increases significantly upon solvation of even a small amount of ionizable material, such as [[hydrogen chloride]]. Thus the risks of [[electric shock|electrocution]] are much greater in water with the usual impurities not found in pure water. Any electrical properties observable in water are from the [[ion]]s of mineral salts and [[carbon dioxide]] dissolved in it. | ||
| − | + | Some molecules of water [[Self-ionization of water|dissociate into ions]], producing [[hydroxide]] anions and [[hydronium]] cations, as noted earlier. This dissociation is at a very low level in pure water, so the water will not carry enough [[electric current]] to do any work or cause any harm for most operations. In pure water, sensitive equipment can detect a very slight electrical conductivity of 0.055 [[Siemens (unit)|µS]]/[[Centimeter|cm]] at 25 °C. Water can also be [[electrolysis|electrolyzed]] into oxygen and hydrogen gases, but in the absence of dissolved ions this is a very slow process, as very little current is conducted. | |
| − | + | === Water containing deuterium and tritium === | |
| + | Hydrogen has three [[isotope]]s. The most common isotope, present in more than 95 percent of water, has 1 proton and no neutron in the atomic nucleus. A second isotope, [[deuterium]] (or "D"), has 1 proton and 1 neutron. Water that contains deuterium ({{chem|D|2|O}}) is also known as [[heavy water]] and is used in [[nuclear reactor]]s for storing nuclear wastes. The third isotope, [[tritium]] (or "T"), has 1 proton and 2 neutrons in the atomic nucleus, and is [[radioactive]]. Water that contains tritium ({{chem|T|2|O}}) does not exist in nature, as the creation of the molecule would result in its almost instantaneous decomposition. {{chem|D|2|O}} is stable, but it differs from {{chem|H|2|O}} in being denser. Also, it can block alpha and beta rays. {{chem|D|2|O}} occurs naturally in water at very low concentrations. Consumption of pure isolated {{chem|D|2|O}} adversely affects biochemical processes: ingestion of large amounts impairs kidney and central nervous system functions. | ||
| − | == | + | ===Heat capacity and heat of vaporization=== |
| + | Water has the second highest [[specific heat capacity]] of any known chemical compound, after [[ammonia]]. In addition, it has a high [[heat of vaporization]] (40.65 kJ mol<sup>−1</sup>). Both these properties are a result of the extensive [[hydrogen bond]]ing between its molecules. These two unusual properties allow water to moderate Earth's [[climate]] by buffering large fluctuations in temperature. | ||
| − | [[ | + | ===Ice floats on liquid water=== |
| + | A simple but environmentally important and unusual property of water is that its solid form, [[ice]], floats on its liquid form, because ice has a lower [[density]] than liquid water. By contrast, for almost all other substances, the solid form has a higher density than the liquid form. This property of water can be explained as follows. | ||
| − | + | When freshwater is cooled, it increases in density, and the cooler water sinks below the warmer layers by [[convection]]. This continues until the water reaches a temperature of 3.98 °C (at standard atmospheric pressure), at which stage water reaches its highest density. Further cooling lowers the density of water, because of the geometry of the hydrogen bonds formed between the molecules. When some of the water freezes, the ice that is formed floats because of its lower density. | |
| − | + | When a body of water such as a lake begins to freeze, ice forms first at the surface and progresses downward. Water in the deeper regions of the lake remains warmer than that near the top. The layer of ice at the top effectively insulates the lake floor from the cold, protecting the fish and other living organisms from freezing to death. | |
| − | + | Although water freezes at 0 °C (32 °F, 273 K), it can be [[supercooled]] in a fluid state down to its [[nucleation|crystal homogeneous nucleation]] at almost 231 K (−42 °C)<ref>P.G. Debenedetti, and H.E. Stanley, [http://polymer.bu.edu/hes/articles/ds03.pdf Supercooled and Glassy Water]. ''Physics Today'' 56(6) (2003):40–46. Retrieved January 9, 2020.</ref>. [[Ice]] also has a number of more exotic phases not commonly seen. | |
| − | === | + | ===Triple point=== |
| − | :'' | + | {{main|Triple point}} |
| − | + | {| class="wikitable" style="float:right;" | |
| − | + | |+The various triple points of water<ref name=Schleuter>Oliver Schlüter, ''[https://depositonce.tu-berlin.de/handle/11303/1060 Impact of High Pressure — Low Temperature Processes on Cellular Materials Related to Foods]''. ''Technischen Universität Berlin'', 2003. Retrieved January 9, 2020.</ref> | |
| + | !Phases in stable equilibrium | ||
| + | !Pressure | ||
| + | !Temperature | ||
| + | |- | ||
| + | |liquid water, [[ice I]], and water vapour | ||
| + | |611.73 Pa | ||
| + | |273.16 K | ||
| + | |- | ||
| + | |liquid water, [[ice Ih]], and [[ice III]] | ||
| + | |209.9 MPa | ||
| + | |251 K (-22 °C) | ||
| + | |- | ||
| + | |liquid water, ice Ih, and gaseous water | ||
| + | |612 Pa | ||
| + | |0.01 °C | ||
| + | |- | ||
| + | |liquid water, ice III, and [[ice V]] | ||
| + | |350.1 MPa | ||
| + | | -17.0 °C | ||
| + | |- | ||
| + | |liquid water, ice V, and [[ice VI]] | ||
| + | |632.4 MPa | ||
| + | |0.16 °C | ||
| + | |- | ||
| + | |ice Ih, [[Ice II]], and ice III | ||
| + | |213 MPa | ||
| + | | -35 °C | ||
| + | |- | ||
| + | |ice II, ice III, and ice V | ||
| + | |344 MPa | ||
| + | | -24 °C | ||
| + | |- | ||
| + | |ice II, ice V, and ice VI | ||
| + | |626 MPa | ||
| + | | -70 °C | ||
| + | |} | ||
| + | The [[triple point]] of water is the combination of pressure and temperature at which pure liquid water, ice, and water vapor can coexist in a stable equilibrium. The [[phase diagram]] of water has several triple points, of which the most familiar one is used to define the [[kelvin]] (K), the SI unit of thermodynamic temperature. As a consequence, this triple point temperature is a prescribed value rather than a measured quantity: 273.16 K (0.01 °C) and a pressure of 611.73 pascals (approximately 0.0060373 [[atmosphere (unit)|atm]]). This triple point is approximately the combination that exists at 100 percent relative humidity at [[sea level]] and the freezing point of water. | ||
| − | + | Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early twentieth century. Kamb and others documented further triple points in the 1960s.<ref>Gustav Tammann ''The States Of Aggregation.'' (Constable And Company Limited, 1925).</ref><ref name=Schleuter /><ref>William Cudmore McCullagh Lewis, and James Rice, ''A System of Physical Chemistry.'' (London, UK: Longmans, Green and co., 1922).</ref> | |
| − | + | ===Miscibility, condensation, and relative humidity=== | |
| + | Water is [[miscible]] with many liquids, for example [[ethanol]] in all proportions, forming a single homogeneous liquid. On the other hand water and most [[oil]]s are ''immiscible'' usually forming layers according to increasing density from the top. | ||
| − | + | [[Image:Relative Humidity.png|thumb|225px|right|Red line shows saturation]] | |
| − | + | As a gas, water vapor is completely [[miscible]] with air. On the other hand the maximum water vapor pressure that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For example, if the vapor ''[[partial pressure]]''<ref>The pressure due to water vapor in the air is called the '''partial pressure'''(Dalton's law) and it is directly proportional to concentration of water molecules in air (Boyle's law).</ref> is 2 percent of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C water will start to condense, defining the [[dew point]], and creating [[fog]] or [[dew]]. The reverse process accounts for the fog ''burning off'' in the morning. | |
| − | + | If one raises the humidity at room temperature, say by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change, and condenses out as steam. | |
| − | + | A gas in this context is referred to as ''saturated'' or 100 percent relative humidity, when the vapor pressure of water in the air is at the equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in air is small, ''relative humidity,'' the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful. | |
| − | + | Water vapor pressure above 100 percent relative humidity is called ''super-saturated'' and can occur if air is rapidly cooled, say by rising suddenly in an updraft.<ref>Adiabatic cooling resulting from the ideal gas law.</ref> | |
| − | Water is | ||
| − | Water is | + | ==Water on Earth== |
| + | {{readout||right|250px|Water covers about 71 percent of the [[Earth]]'s surface}} | ||
| + | Water is found in a variety of locations on Earth, in solid, liquid, and gaseous states. Accordingly, it is known by different names: [[water vapor]] and [[cloud]]s in the sky; [[seawater]] and [[iceberg]]s in the ocean; [[glacier]]s and rivers in the [[mountain]]s; and [[aquifer]]s in the ground. About 1,460 [[Tonne#Multiples|teratonnes]] (Tt)<ref>One tonne (or metric ton) is defined as 1000 [[kilogram]]s (kg) or 1 megagram (Mg). One teratonne is equal to 10<sup>12</sup> tonnes.</ref> of water covers about 71 percent of the [[Earth]]'s surface. [[Seawater|Saltwater]] [[ocean]]s hold 97 percent of surface water, [[glacier]]s and polar [[ice cap]]s 2.4 percent, and other land surface water such as [[river]]s and [[lake]]s 0.6 percent. | ||
| − | + | ===Origin and planetary effects=== | |
| + | [[File:Gliese 581 - 2010.jpg|thumb|300px|right|Planetary habitable zones of the Solar System and the Gliese 581 system compared]] | ||
| − | + | It is thought that much of the universe's water may have been produced as a by-product of [[star formation]]. The birth of a star is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the resultant shock waves compress and heat the gas. Water could be quickly produced in this warm, dense gas.<ref>Gary Melnick and David Neufeld, [http://pages.jh.edu/~news_info/news/home98/apr98/clouds.html Space Cloud Holds Enough Water to Fill Earth's Oceans 1 Million Times], ''Headlines@Hopkins, JHU'', 1998. Retrieved January 9, 2020.</ref> | |
| − | == | + | === Earth's habitability === |
| + | The existence of liquid water, and to a lesser extent its gaseous and solid forms, on Earth is vital to the existence of [[life on Earth]]. The Earth is located in the [[habitable zone]] of the [[Solar System]]. If it were slightly closer to or farther from the [[Sun]] (about 5 percent, or 8 million kilometers or so), the conditions that allow the three forms of water to be present simultaneously would be far less likely to prevail.<ref>E. Ehlers and T. Krafft (eds.), ''Understanding the Earth System: compartments, processes, and interactions.'' (New York, NY: Springer, 2001).</ref><ref>[http://www.daviddarling.info/encyclopedia/H/habzone.html Habitable Zone]. ''The Encyclopedia of Astrobiology, Astronomy and Spaceflight.'' Retrieved January 9, 2020.</ref> | ||
| − | + | Earth's mass allows its [[gravity]] to hold an [[Celestial body atmosphere|atmosphere]]. Water vapor and carbon dioxide in the atmosphere provide a [[greenhouse effect]] that helps maintain a relatively steady surface temperature. If Earth were smaller, a thinner atmosphere would cause temperature extremes, preventing the accumulation of water except at the [[polar ice cap]]s (as on [[Mars (planet)|Mars]]). If Earth were too massive, the water on it could have been in the solid state even at relatively high temperatures, because of the high pressure caused by gravity. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | It has been proposed that life itself may maintain the conditions that have allowed its continued existence. Earth's surface temperature has been relatively constant through [[geologic time]], despite varying levels of incoming solar radiation ([[insolation]]), indicating that a dynamic process governs Earth's temperature via a combination of greenhouse gases and surface or atmospheric [[albedo]]. This proposal is known as the ''[[Gaia hypothesis]].'' | |
| − | {{ | + | ===Tides=== |
| − | {{ | + | {{imageframe |
| − | + | |width=300 | |
| − | + | |content=[[Image:Bay of Fundy High Tide.jpg|150px]][[Image:Bay of Fundy Low Tide.jpg|150px]] | |
| − | + | |caption=High tide (left) and low tide (right). | |
| − | + | }} | |
| − | + | {{main|Tide}} | |
| − | + | '''[[Tide]]s''' are the cyclic rising and falling of [[Earth]]'s [[ocean]] surface caused by the [[tidal force]]s of the [[Moon]] and the [[Sun]] acting on the oceans. Tides cause changes in the depth of the marine and [[estuary|estuarine]] water bodies and produce oscillating currents known as tidal streams. | |
| − | + | The changing tide produced at a given location is the result of the changing positions of the Moon and Sun relative to the Earth coupled with the [[Coriolis effect|effects of Earth rotation]] and the local [[bathymetry]]. | |
| − | + | The strip of seashore that is submerged at high tide and exposed at low tide, the [[intertidal zone]], is an important ecological product of ocean tides. | |
| − | + | ||
| − | + | === Water cycle === | |
| − | + | The [[biosphere]] can be roughly divided into oceans, land, and atmosphere. Water moves perpetually through each of these regions in the ''[[water cycle]],'' which consists of following transfer processes: | |
| − | + | *[[evaporation]] from oceans and other water bodies into the air and [[transpiration]] from land plants and animals into air. | |
| − | + | *[[precipitation (meteorology)|precipitation]], from water vapor condensing from the air and falling to earth or ocean. | |
| − | + | *[[runoff (water)|runoff]] from the land usually reaching the [[sea]]. | |
| − | + | ||
| − | + | [[Image:WhereRainbowRises.jpg|right|200px|thumb|Rain [[refract]]s [[sunlight]] to produce this [[rainbow]].]] | |
| − | + | ||
| − | + | Most water vapor over the oceans returns to the oceans, but winds carry water vapor over land at the same rate as runoff into the sea, about 36 [[Tonne#Multiples|Tt]] per year. Over land, evaporation and transpiration contribute another 71 Tt per year. Precipitation, at a rate of 107 Tt per year over land, has several forms: most commonly [[rain]], [[snow]], and [[hail]], with some contribution from [[fog]] and [[dew]]. | |
| − | + | Condensed water in the air may also [[refract]] [[sunlight]] to produce [[rainbow]]s. | |
| − | + | ||
| − | + | Water runoff often collects over [[Drainage basin|watershed]]s flowing into rivers. Some of this is diverted to [[irrigation]] for [[agriculture]]. Rivers and seas offer opportunity for [[travel]] and [[commerce]]. Through [[erosion]], runoff shapes the environment creating river [[valley]]s and [[river delta|deltas]] that provide rich soil and level ground for the establishment of population centers. | |
| − | + | ||
| + | ===Freshwater storage=== | ||
| + | Some runoff water is trapped for periods, for example in [[lake]]s. In addition, snow and ice collect at the poles, on high mountains, and in other regions that experience cold winters. Water also infiltrates the ground and goes into aquifers. This [[groundwater]] later flows back to the surface in [[spring (hydrosphere)|springs]], or more spectacularly in [[hot spring]]s and [[geyser]]s. Groundwater may be extracted artificially by digging [[water well|well]]s. | ||
| + | |||
| + | These forms of water storage are important because clean, freshwater is essential for [[human]] and other land-based life forms. In many parts of the world, freshwater is in short supply. | ||
| + | |||
| + | [[Image:SnowflakesWilsonBentley.jpg|right|thumb|200px|''Snowflakes'' by [[Wilson Bentley]], 1902.]] | ||
| + | |||
| + | ===Tastes and odors of water=== | ||
| + | Given that water can dissolve many different substances, it acquires different tastes and odors. In fact, humans and animals have developed senses to be able to evaluate the [[drinking water|potability]] of water. Animals generally dislike the taste of [[salt]]y [[sea water]] and the putrid [[swamp]]s and favor the purer water of a mountain spring or aquifer. The taste advertised in [[spring water]] or [[mineral water]] derives from the minerals dissolved in it, as pure H<sub>2</sub>O is tasteless. The "[[purity]]" of spring and mineral water refers to the absence of [[toxin]]s, [[pollutant]]s, and harmful [[microorganism|microbe]]s. | ||
| + | |||
| + | == Effects on life == | ||
| + | [[Image:Blue Linckia Starfish.JPG|thumb|220px|right|Some of the [[biodiversity]] of a [[coral reef]].]] | ||
| + | |||
| + | Water has many distinct properties that are critical for the proliferation of all known forms of [[life]], setting it apart from other substances. It is vital both as a [[solvent]] in which many of the body's solutes dissolve and as an essential part of many [[metabolism|metabolic]] processes within the body, including reactions that lead to cellular [[replication]] and growth. | ||
| + | |||
| + | Metabolism is the sum total of anabolism and catabolism. In anabolism, water is removed from molecules (through energy-requiring enzymatic reactions) to build larger molecules (such as starches, triglycerides, and proteins for storage of fuels and information). In catabolism, water is used to break bonds, to generate smaller molecules (such as glucose, fatty acids, and amino acids). Water is thus essential and central to these metabolic processes. Without water, these metabolic processes would cease to exist. | ||
| + | |||
| + | Biochemical reactions take place in water at specific pH values. For instance, human enzymes usually perform optimally around a pH of 7.4. Digestion of food in the stomach requires the activity of an acid (hydrochloric acid, HCl). Some people suffer from what is called "acid reflux," in which the stomach acid makes its way into and adversely affects the esophagus. This condition can be temporarily neutralized by ingestion of a base such as [[aluminum hydroxide]] to produce the neutral molecules of water and aluminum chloride (a salt). | ||
| + | |||
| + | Water is also central to photosynthesis and respiration. Photosynthetic cells use the Sun's energy to split off water's hydrogen from oxygen. Hydrogen is combined with carbon dioxide (absorbed from air or water) to form glucose and release oxygen. All living cells use such fuels and oxidize the hydrogen and carbon to capture the Sun's energy and reform water and carbon dioxide in the process (cellular respiration). | ||
| + | |||
| + | ===Aquatic life forms=== | ||
| + | [[Image:Diatoms through the microscope.jpg|thumb|250px|right|Some marine [[diatom]]s - a key [[phytoplankton]] group.]] | ||
| + | |||
| + | Earth's waters are filled with life. Nearly all [[fish]] live exclusively in water, and many marine mammals, such as [[dolphin]]s and [[whale]]s, also live in the water. Some kinds of animals, such as [[amphibian]]s, spend portions of their lives in water and portions on land. Plants such as [[kelp]] and [[algae]] grow in the water and are the basis for some underwater ecosystems. [[Plankton]] is generally the foundation of the ocean food chain. | ||
| + | |||
| + | Different water creatures use different ways of obtaining oxygen in the water. Fish have [[gill]]s instead of [[lung]]s, although some species of fish, such as the [[lungfish]], have both. [[Marine mammal]]s, such as dolphins, whales, [[otter]]s, and [[pinniped|seals]], need to surface periodically to breathe air. | ||
| + | |||
| + | == Human uses == | ||
| + | [[Civilization]] has historically flourished around [[river]]s and major waterways. [[Mesopotamia]], the so-called cradle of civilization, was situated between the major rivers [[Tigris]] and [[Euphrates]]; the ancient [[Egyptians]] depended greatly upon the [[Nile]]. Large metropolitan areas like [[Rotterdam]], [[London]], [[Montreal]], [[Paris]], [[New York City]], [[Shanghai]], [[Tokyo]], [[Chicago]], [[Mumbai]], and [[Hong Kong]] owe their success in part to their easy accessibility via water and the resultant expansion of trade. Islands with safe water ports, like [[Singapore]], have flourished for the same reason. In regions such as [[North Africa]] and the [[Middle East]], where freshwater is relatively scarce, access to clean drinking water has been a major factor in human development. | ||
| + | |||
| + | Water fit for [[human]] consumption is called [[drinking water]] or [[potable water]]. Water that is not potable can be made potable by various methods, including: [[filtration]], to remove particulate impurities; chemical or heat treatment, to kill bacteria; and [[distillation]], to separate water from impurities by vaporization and condensation. It should be noted, however, that some [[solute]]s in potable water are acceptable and even desirable for taste enhancement and to provide needed [[electrolyte]]s. | ||
| + | |||
| + | Water that is not fit for drinking but is not harmful if used for swimming or bathing is sometimes called "safe water" or "safe for bathing." Chlorine, a skin and mucous membrane irritant, is used to make water safe for bathing or drinking. Its use is highly technical and is usually monitored by government regulations (typically 1 part per million (ppm) for drinking water, and 1-2 ppm of chlorine not yet reacted with impurities for bathing water). | ||
| + | |||
| + | The single largest freshwater resource suitable for drinking is [[Lake Baikal]] in [[Siberia]], which has a very low [[salt]] and [[calcium]] content and is very clean. | ||
| + | |||
| + | === Drinking water === | ||
| + | [[Image:TapWater-china.JPG|thumb|right|250px|A manual water [[pump]] in China.]] | ||
| + | |||
| + | About 70 percent of the fat-free mass of the [[human]] body is made of water. To function properly, the body requires between one and seven [[liter]]s of water per [[day]] to avoid [[dehydration]]; the precise amount depends on the level of activity, temperature, humidity, and other factors. Most of this is ingested through foods or beverages other than drinking straight water. It is not clear how much water intake is needed by healthy people. | ||
| + | |||
| + | For those who have healthy [[kidney]]s, it is rather difficult to drink too much water, but (especially in warm humid weather and while exercising) it is dangerous to drink too little. People can drink far more water than necessary while exercising, however, putting them at risk of [[water intoxication]], which can be fatal. The "fact" that a person should consume eight glasses of water per day cannot be traced back to a scientific source.<ref>Heinz Valdin, [https://www.physiology.org/doi/full/10.1152/ajpregu.00365.2002 "Drink at least eight glasses of water a day." Really? Is there scientific evidence for "8 × 8"?] ''Department of Physiology, Dartmouth Medical School''. Retrieved January 9, 2020.</ref> There are other [[myth]]s such as the effect of water on weight loss and constipation that have been dispelled. | ||
| + | |||
| + | Original recommendation for water intake in 1945 by the [[Food and Nutrition Board]] of the [[National Research Council]] read: "An ordinary standard for diverse persons is 1 milliliter for each calorie of food. Most of this quantity is contained in prepared foods."<ref>Food and Nutrition Board, National Academy of Sciences. Recommended Dietary Allowances, revised 1945. ''National Research Council, Reprint and Circular Series''. 122:3-18.</ref> The latest dietary reference intake report by the [[United States National Research Council]] in general recommended (including food sources): 2.7 liters of water total for women and 3.7 liters for men.<ref>[https://www.nap.edu/read/10925/chapter/6 Dietary Reference Intakes: Water, Potassium, Sodium, Chloride, and Sulfate]. Food and Nutrition Board, ''National Institute of Medicine'', 2005. Retrieved January 9, 2020.</ref> Specifically, [[Pregnancy|pregnant]] and [[breastfeeding]] women need additional fluids to stay hydrated. According to the [[Institute of Medicine]]—who recommend that, on average, women consume 2.2 liters and men 3.0 liters—this is recommended to be 2.4 liters (approx. 9 cups) for pregnant women and 3 liters (approx. 12.5 cups) for breastfeeding women, since an especially large amount of fluid is lost during nursing.<ref>[https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/water/art-20044256 Water: How much should you drink every day?] ''Mayo Clinic''. Retrieved January 9, 2020.</ref> Also noted is that, normally, about 20 percent of water intake comes from food, while the rest comes from drinking water and beverages ([[Caffeine|caffeinated]] included). Water is excreted from the body in multiple forms: through [[urine]], [[feces]], [[sweat]]ing, and exhalation of [[water vapor]] in the breath. With physical exertion and heat exposure, water loss will increase and daily fluid needs may increase as well. | ||
| + | |||
| + | ===Agriculture=== | ||
| + | In many developing nations, irrigation accounts for over 90 percent of water withdrawn from available sources for use. In England, where rain is abundant year round, water used for agriculture accounts for less than 1 percent of human usage. Yet even on the same continent, water used for irrigation in Spain, Portugal and Greece exceeds 70 percent of total usage. | ||
| + | |||
| + | Irrigation has been a key component of the "[[green revolution]]," which has enabled many developing countries to produce enough food to feed everyone. More water will be needed to produce more food for 3 billion more people. But increasing competition for water and inefficient irrigation practices could constrain future food production. | ||
| + | |||
| + | ===As a cleaning agent=== | ||
| + | Water is important for washing the human body and everyday items such as clothes, floors, cars, food, and pets. | ||
| + | |||
| + | === Standard of measurement === | ||
| + | On April 7, 1795, the [[gram]] was defined in [[France]] to be equal to "the absolute weight of a volume of pure water equal to a cube of one hundredth of a meter, and to the temperature of the melting ice." For practical purposes though, a metallic reference standard was required, one thousand times more massive, the [[kilogram]]. Work was therefore commissioned to determine precisely how massive one [[Litre|liter]] of water was. In spite of the fact that the decreed definition of the gram specified water at 0 °C—a highly stable ''temperature'' point—the scientists chose to redefine the standard and to perform their measurements at the most stable ''density'' point: the temperature at which water reaches maximum density, which was measured at the time as 4 °C. | ||
| + | |||
| + | ===As a thermal transfer agent=== | ||
| + | [[Boiling]], [[steaming]], and [[simmering]] are popular [[cooking]] methods that often require immersing food in water or its gaseous state, steam. Water is also used in industrial contexts as a [[coolant]], and in almost all power-stations as a coolant and to drive steam [[turbine]]s to generate electricity. In the nuclear industry, water can also be used as a [[neutron moderator]]. | ||
| + | |||
| + | ===Recreation=== | ||
| + | Humans use water for many recreational purposes, as well as for exercising and sports. Some of these include [[swimming]], [[waterskiing]], [[boating]], [[fishing]], and [[diving]]. In addition, some sports, like [[ice hockey]] and [[ice skating]], are played on ice. Likewise, sports such as [[skiing]] or [[snowboarding]] require the water to be frozen. Many use water for [[play fighting]], such as with [[snowball]]s, [[water gun]]s, or [[water balloon]]s. | ||
| + | |||
| + | Lakesides and beaches are popular places for people to go for recreation and relaxation. Many find the sound of flowing water to be calming. Some keep fish and other life in [[aquarium|water tanks]] or [[pond]]s for show, fun, and companionship. People also make fountains and use water in their public or private decorations. | ||
| + | |||
| + | ===Industrial applications=== | ||
| + | Pressurized water is used in [[water blasting]] and [[water jet cutter]]s. Also, high-pressure water guns are used for precise cutting. It is also an effective coolant for various [[machine]]s that generate heat during operation. It works very well, is relatively safe, and is not harmful to the environment. | ||
| + | |||
| + | ===Food processing=== | ||
| + | Water plays many critical roles within the field of [[food science]]. Food scientists need to understand the roles of water in food processing, to ensure the success of their products. | ||
| + | |||
| + | Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water is affected by solutes. One [[mole (unit)|mole]] of sucrose (sugar) raises the boiling point of water by 0.52 °C, and one mole of salt raises the boiling point by 1.04 °C while lowering the freezing point of water in a similar way.<ref name="vaclacik"> Vickie A. Vaclavik and Elizabeth W. Christian, ''Essentials of Food Science'' (New York, NY: Kluwer Academic/Plenum Publishers, 2003, ISBN 0306473631).</ref> Solutes in water also affect water activity which affects many chemical reactions and the growth of microbes in food.<ref name="deman">John M. DeMan, ''Principles of Food Chemistry'' (Gaithersburg, MD: Aspen Publishers, 1999, ISBN 083421234X).</ref> Water activity can be described as a ratio of the vapor pressure of water in a solution to the vapor pressure of pure water.<ref name="vaclacik"/> Solutes in water lower water activity. This is important to know because most bacterial growth ceases at low levels of water activity.<ref name="deman" /> Not only does microbial growth affect the safety of food but also the preservation and shelf life of food. | ||
| + | |||
| + | Water hardness is also a critical factor in food processing. It can dramatically affect the quality of a product as well as playing a role in sanitation. Water hardness is classified based on the amounts of removable calcium carbonate salt it contains per gallon. Water hardness is measured in grains; 0.064 g calcium carbonate is equivalent to one grain of hardness.<ref name="vaclacik"/> Water is classified as soft if it contains 1 to 4 grains, medium if it contains 5 to 10 grains and hard if it contains 11 to 20 grains.<ref name="vaclacik"/> The hardness of water may be altered or treated by using a chemical ion exchange system. The hardness of water also affects its pH balance which plays a critical role in food processing. For example, hard water prevents successful production of clear beverages. Water hardness also affects sanitation; with increasing hardness, there is a loss of effectiveness for its use as a sanitizer.<ref name="vaclacik"/> | ||
| + | |||
| + | ===Power generation=== | ||
| + | [[Hydroelectricity]] is electricity obtained from [[hydropower]]. Hydroelectric power comes from water driving a turbine connected to a generator. Hydroelectricity is a low-cost, non-polluting, renewable energy source. | ||
| + | |||
| + | == Water resource distribution and pollution == | ||
| + | [[Image:Evstafiev-bosnia-sarajevo-water-line.jpg|thumb|left|250px|People wait in line to gather water, during the [[Siege of Sarajevo]].]] | ||
| + | |||
| + | Water in itself is not a finite resource (like petroleum is). The water cycle, which involves evaporation, condensation, and precipitation, regenerates potable water in large quantities, many orders of magnitude higher than human consumption. However, many parts of the world are experiencing water scarcity, in the sense that there are problems with the distribution of potable and irrigation water. Such shortages of water form a major social and economic concern and have led to disputes between nations that rely on the same source of water (such as the same river). Some countries experiencing water shortages import water or purify seawater by [[desalination]]. | ||
| + | |||
| + | Currently, about 1 billion people around the world routinely drink unhealthy water. Poor water quality and bad sanitation are deadly; some 5 million deaths a year are caused by polluted drinking water. | ||
| + | |||
| + | In the developing world, 90 percent of all [[wastewater]] goes untreated into local rivers and streams. Some 50 countries, with roughly a third of the world’s population, also suffer from medium or high water stress, and a number of them extract more water annually than is recharged through their natural water cycles. The strain affects surface freshwater bodies like rivers and lakes, but it also degrades groundwater resources. | ||
| + | |||
| + | Water is a strategic resource in the globe and an important element in many political conflicts. Some have predicted that clean water will become the "next oil," making [[Canada]], with this resource in abundance, possibly the richest country in the world. There is a long history of conflict over water, including efforts to gain access to water, the use of water in wars started for other reasons, and tensions over shortages and control.<ref>[http://www.worldwater.org/water-conflict/ A Chronology of Water-Related Conflicts] ''The World's Water'', Pacific Institute. Retrieved January 9, 2020.</ref> | ||
| + | |||
| + | [[UNESCO]]'s World Water Development Report (WWDR, 2003) from its [[World Water Assessment Program]] indicates that, in the next 20 years, the quantity of water available to everyone is predicted to decrease by 30 percent. About 40 percent of the world's inhabitants currently have insufficient fresh water for minimal [[hygiene]]. More than 2.2 million people died in 2000 from [[disease]]s related to the consumption of contaminated water or [[drought]]. In 2004, the UK charity [[WaterAid]] reported that a child dies every 15 seconds from easily preventable water-related diseases; often this means lack of [[sewage]] disposal; see [[toilet]]. | ||
| + | |||
| + | === Water availability in specific regions === | ||
| + | Ninety-five percent of freshwater in the United States is [[underground]]. One crucial source is a huge underground [[reservoir]], the 1,300-kilometer (800 mi) [[Ogallala Aquifer|Ogallala aquifer]] which stretches from [[Texas]] to [[South Dakota]] and waters one fifth of U.S. irrigated land. Formed over millions of years, the Ogallala aquifer has since been cut off from its original natural sources. It is being depleted at a rate of 12 billion cubic meters (420 billion ft<sup>3</sup>) per year, amounting to a total depletion to date of a volume equal to the annual flow of 18 [[Colorado River]]s. Some estimates say it will dry up in as little as 25 years. Many farmers in the [[High Plains (United States)|Texas High Plains]], which rely particularly on the underground source, are now turning away from [[irrigated agriculture]] as they become aware of the hazards of overpumping.<ref>[http://news.bbc.co.uk/1/shared/spl/hi/world/03/world_forum/water/html/ogallala_aquifer.stm Ogallala aquifer - Water hot spots]. ''BBC News''. Retrieved January 9, 2020.</ref> | ||
| + | |||
| + | The [[Middle East]] region has only 1 percent of the world's available freshwater, which is shared among 5 percent of the world's population. Thus, in this region, water is an important strategic resource. It is predicted that by 2025, countries of the Arabian peninsula will be using more than twice the amount of water naturally available to them.<ref>Ben Sutherland, [http://news.bbc.co.uk/1/hi/sci/tech/2859937.stm Water shortages 'foster terrorism'] ''BBC News'', March 18, 2003. Retrieved January 9, 2020.</ref> According to a report by the [[Arab League]], two-thirds of Arab countries have less than 1,000 cubic meters (35,000 ft<sup>3</sup>) of water per person per year available, which is considered the limit.<ref>Christian Chesnot, "Major aspects of scarce water resources management with reference to the Arab countries," Arab League report published for the International Conference on water gestion and water politics in arid zones, in Amman, Jordan, December 1-3, 1999. in ''Drought in the Middle East.'' (Monde diplomatique.)</ref> | ||
| + | |||
| + | [[Image:200407-sandouping-sanxiadaba-4.med.jpg|thumb|300px|Three Gorges Dam, receiving, upstream side, July 26, 2004]] | ||
| + | |||
| + | In [[Asia]], [[Cambodia]] and [[Vietnam]] are concerned about attempts by [[China]] and [[Laos]] to control the flux of water. China is preparing the [[Three Gorges Dam]] project on the [[Yangtze River]], which would become the world's largest [[dam]], causing many social and environmental problems. It also has a project to divert water from the Yangtze to the dwindling [[Yellow River]], which feeds China's most important farming region. | ||
| + | |||
| + | [[Image:Ganges River Delta, Bangladesh, India.jpg|thumb|225px|right|Ganges [[river delta]], Bangladesh and India]] | ||
| + | |||
| + | The [[Ganges]] is disputed between [[India]] and [[Bangladesh]]. The water reserves are being quickly depleted and polluted, while the [[glacier]] feeding the sacred [[Hinduism|Hindu]] river is retreating hundreds of feet each year, causing subsoil streams flowing into the Ganges river to dry up. | ||
| + | |||
| + | In [[South America]], the [[Guaraní Aquifer]] is located between the [[Mercosur]] countries of [[Argentina]], [[Brazil]], [[Bolivia]] and [[Paraguay]]. With a volume of about 40,000 km³, it is an important source of fresh potable water for all four countries. | ||
| + | |||
| + | === Purification and waste reduction === | ||
| + | Drinking water is often collected at [[spring (hydrosphere)|springs]], extracted from artificial [[Boring (mechanical)|borings]] in the ground, or wells. Building more wells in adequate places is thus a possible way to produce more water, assuming the aquifers can supply an adequate flow. Other water sources are rainwater and river or lake water. This surface water, however, must be [[water purification|purified]] for human consumption. This may involve removal of undissolved substances, dissolved substances and harmful [[microbe]]s. Popular methods are [[filter (water)|filtering]] with sand which only removes undissolved material, while [[chlorination]] and [[boiling]] kill harmful microbes. [[Distillation]] does all three functions. More advanced techniques are also available, such as [[reverse osmosis]]. [[Desalination]] of [[seawater]] is a more expensive solution, but it is used in some coastal areas with [[arid]] [[climate]]s because the water is abundantly available. | ||
| + | |||
| + | The distribution of drinking water is done through [[municipal water system]]s or as [[bottled water]]. Governments in many countries have programs to distribute water to the needy at no charge. Others argue that the [[market]] mechanism and [[free enterprise]] are best to manage this rare resource and to finance the boring of wells or the construction of dams and [[reservoir (water)|reservoirs]]. | ||
| + | |||
| + | Reducing waste by using drinking water only for human consumption is another option. In some cities such as [[Hong Kong]], seawater is extensively used for flushing toilets to conserve freshwater resources. | ||
| + | |||
| + | Polluting water may be the biggest single misuse of water; to the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. Like other types of pollution, this does not enter standard accounting of market costs, being conceived as [[externality|externalities]] for which the market cannot account. Thus other people pay the price of water pollution, while the private firms' profits are not redistributed to the local people who are victims to this pollution. [[Pharmaceutical]]s consumed by humans often end up in the waterways and can have detrimental effects on [[marine biology|aquatic]] life if they [[bioaccumulation|bioaccumulate]]. | ||
| + | |||
| + | == Religion and philosophy == | ||
| + | [[Image:Hindu water ritual.jpg|thumb|250px|A Hindu ablution as practiced in [[Tamil Nadu]].]] | ||
| + | |||
| + | In most religions, water is considered a purifier in an internal, spiritual sense as well as in an external, physical sense. Faiths that incorporate ritual washing ([[ablution]]) include [[Hinduism]], [[Christianity]], [[Islam]], [[Judaism]], [[Zoroastrianism]], and [[Shinto]]. Water is mentioned in the [[Bible]] 442 times in the [[New International Version]] and 363 times in the [[King James Version]]. For example, 2 Peter 3:5(b) states, "The earth was formed out of water and by water" (NIV). | ||
| + | |||
| + | Water [[baptism]] is a central [[sacrament]] of Christianity. It is also a part of the practice of other religions, including Judaism ''([[mikvah]])'' and [[Sikhism]] ''([[Amrit Sanskar]]).'' In [[Zoroastrianism]], one is expected to wash one's hands and face before praying in the [[fire temple]]. Likewise, in [[Islam]], the five daily prayers can be offered in [[Tayammum|most cases]] after washing certain parts of the body with clean water ''([[wudu]]).'' In [[Shinto]], water is used in almost all rituals to cleanse a person or area (such as in the ritual of ''[[misogi]]''). In addition, a ritual bath in pure water is performed for the [[deceased|dead]] in many religions, including Judaism and Islam. | ||
| + | |||
| + | Some faiths use water especially prepared for religious purposes—[[holy water]] in some Christian denominations; ''[[Amrit]]'' in Sikhism and Hinduism. Many religions also consider particular sources or bodies of water to be sacred or at least auspicious. Examples include [[Lourdes]] in [[Roman Catholicism]], the [[Zamzam Well]] in Islam, and the River [[Ganges]] (among many others) in Hinduism. In Neo-Paganism water is often combined with salt in the first steps of a ritual, to act as a purifier of worshippers and the altar, symbolizing both cleansing tears and the ocean. | ||
| + | |||
| + | Water is often believed to have spiritual powers. In [[Celtic mythology]], [[Sulis]] is the local [[goddess]] of thermal springs; in [[Hinduism]], the [[Ganga in Hinduism|Ganges]] is also personified as a goddess, while [[Saraswati]] has been referred to as a goddess in [[Veda]]s. Also water is one of the "panch-tatva"s (basic 5 elements, others including [[fire]], [[earth]], [[space]], [[air]]). | ||
| + | |||
| + | Alternatively, gods can be patrons of particular springs, rivers, or lakes. For example, in [[Greek mythology|Greek]] and [[Roman mythology|Roman]] [[mythology]], [[Peneus]] was a river god, one of the three thousand [[Oceanid]]s. In [[Islam]], not only does water give life, but every life is itself made of water: "We made from water every living thing".<ref>[[Sura]] of [[Al-Anbiya]] 21:30</ref> | ||
| + | |||
| + | The [[Ancient Greece|Greek]] [[philosopher]] [[Empedocles]] held that water is one of the four [[classical element]]s along with [[fire]], [[earth]] and [[Air (classical element)|air]], and was regarded as the [[ylem]], or basic substance of the universe. Water was considered cold and moist. In the theory of the four [[four humours|bodily humor]]s, water was associated with [[phlegm]]. Water was also one of the [[Five elements (Chinese philosophy)|five elements]] in traditional [[Chinese philosophy]], along with [[earth (classical element)|earth]], [[fire (classical element)|fire]], [[wood (classical element)|wood]], and [[metal (classical element)|metal]]. | ||
| + | |||
| + | == Notes == | ||
| + | <references/> | ||
== References == | == References == | ||
| + | * Anderson, Terry L. ''Water Rights: Scarce Resource Allocation, Bureaucracy, and the Environment.'' Cambridge, MA: Ballinger Pub. Co., 1991. ISBN 0884103900. | ||
| + | * Barlow, Maude, Tony Clarke. ''Blue Gold: The Fight to Stop the Corporate Theft of the World's Water.'' New York, NY: New Press (distributed by W.W. Norton), 2003. ISBN 1565847318. | ||
| + | * de Villiers, Marq. ''Water: The Fate of Our Most Precious Resource.'' Toronto, ON: M&S, 2003. ISBN 0771026412. | ||
| + | * Debenedetti, P.G., and H.E. Stanley. [http://polymer.bu.edu/hes/articles/ds03.pdf Supercooled and Glassy Water]. ''Physics Today'' 56(6) (2003):40–46. Retrieved January 9, 2020. | ||
| + | * DeMan, John M. ''Principles of Food Chemistry 3rd Edition.'' Gaithersburg, MD: Aspen Publishers, 1999. ISBN 083421234X. | ||
| + | * Ehlers, Eckart, and T. Krafft (eds.). ''Understanding the Earth System: compartments, processes, and interactions.'' New York, NY: Springer,2001. ISBN 3540675159. | ||
| + | * Franks, F. (ed.). ''Water, A comprehensive treatise.'' New York, NY: Plenum Press, 1982. ISBN 0306407108. | ||
| + | * Gleick, Peter H. ''The World's Water: The Biennial Report on Freshwater Resources.'' Washington, DC: Island Press, 2006. ISBN 978-1597261050. | ||
| + | * Jones, O.A., J.N. Lester and N. Voulvoulis. Pharmaceuticals: a threat to drinking water? ''TRENDS in Biotechnology'' 23(4) (2005):163. | ||
| + | * Lowi, Miriam R. ''Water and Power: The Politics of a Scarce Resource in the Jordan River Basin.'' New York, NY: Cambridge University Press, 1995. ISBN 0521431646. | ||
| + | * Marks, William E. ''The Holy Order of Water: Healing Earth's Waters and Ourselves.'' Great Barrington, MA: Bell Pond Books (div. of Steiner Books), 2001. ISBN 088010483X | ||
| + | * Postel, Sandra. ''Last Oasis: Facing Water Scarcity.'' New York, NY: Norton Press, 1997. ISBN 0393317447. | ||
| + | * Reisner, Marc. ''Cadillac Desert: The American West and Its Disappearing Water.'' New York, NY: Penguin Books, 1993. ISBN 0140178244. | ||
| + | * Roddick, Anita et al. ''Troubled Water: Saints, Sinners, Truth And Lies About The Global Water Crisis.'' White River Jct., VT: Chelsea Green Publishing Company, 2004. ISBN 095439593X. | ||
| + | * Shiva, Vandana. ''Water Wars: Privatization, Pollution, and Profit.'' Cambridge, MA: South End Press, 2002. ISBN 0745318371. | ||
| + | * Vaclavik, Vickie A. and Elizabeth W. Christian. ''Essentials of Food Science, 2nd Edition.'' New York, NY: Kluwer Academic/Plenum Publishers, 2003. ISBN 0306473631. | ||
| + | * Ward, Diane Raines. ''Water Wars: Drought, Flood, Folly and the Politics of Thirst.'' New York. NY: Riverhead Books, 2002. ISBN 1573222291. | ||
| + | * Worster, Donald. ''Rivers of Empire: Water, Aridity, and the Growth of the American West.'' New York, NY: Pantheon Books, 1992. ISBN 039451680X. | ||
| + | |||
| + | ==External links== | ||
| + | All links retrieved May 3, 2023. | ||
| + | |||
| + | * [http://www.awra.org/ American Water Resources Association]. | ||
| + | * [http://www.wateraid.org/ WaterAid] Charity dedicated to the provision of clean water, sanitation and hygiene education. | ||
| + | * [http://twt.mpei.ac.ru/ochkov/WSPHB/Engindex.html Water and Steam Tables for Industrial Use]. | ||
| − | + | {{BranchesofFoodChemistry}} | |
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
| + | [[Category:Earth sciences]] | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
| − | [[Category: | + | [[Category:Environmental science]] |
| − | [[Category: | + | [[Category:Meteorology]] |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | {{credit| | + | {{credit|161788393}} |
Latest revision as of 23:15, 3 May 2023
- This article is about the chemical substance.
| Water | |
|---|---|
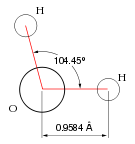 
Water is the base of all life, and | |
| Information and properties | |
| Systematic name | water |
| Alternative names | aqua, dihydrogen monoxide, hydrogen hydroxide, (more) |
| Molecular formula | H2O |
| InChI | InChI=1/H2O/h1H2 |
| Molar mass | 18.0153 g/mol |
| Density and phase | 0.998 g/cm³ (liquid at 20 °C) 0.92 g/cm³ (solid) |
| Melting point | 0 °C (273.15 K) (32 °F) |
| Boiling point | 100 °C (373.15 K) (212 °F) |
| Specific heat capacity | 4.184 J/(g·K) (liquid at 20 °C) |
Water is a common chemical substance that is essential for all known forms of life.[1] In typical usage, the term water refers to its liquid state, but the substance also has a solid state, ice, and a gaseous state, water vapor. About 71 percent of the Earth's surface is covered by water, mostly in oceans and other large water bodies.
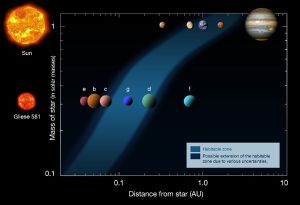
The presence of water on Earth depends on various factors, including the Earth's location in the Solar System. If Earth were about 5 percent closer to or farther from the Sun, there would have been a much lower likelihood for the three forms of water to be present on this planet. Also, the Earth's mass is appropriate for gravity to hold an atmosphere, in which water vapor (along with carbon dioxide) helps maintain a relatively steady surface temperature. A smaller Earth would have a thinner atmosphere, causing temperature extremes and preventing the accumulation of water except at the polar ice caps. If Earth were much more massive, the water on it could have been in the solid state even at relatively high temperatures, because of the high pressure caused by gravity.
Water moves continually through a cycle of evaporation or transpiration, precipitation, and runoff, usually reaching the sea. Winds carry water vapor over land at the same rate as runoff into the sea, about 36 Tt per year. Over land, evaporation and transpiration contribute another 71 Tt per year to the precipitation of 107 Tt per year over land. Some water is trapped for varying periods in ice caps, glaciers, aquifers, or in lakes, sometimes providing freshwater for life on land. Water is a good solvent for a wide variety of substances.
Humans use water for many purposes, including drinking, cooking, cleaning, heating, and cooling. We find it valuable for scientific experimentation and industrial processes as well as for agriculture. In addition, we use water for various sports and recreational activities. In various religions, water is considered a purifier in an internal, spiritual sense as well as in an external, physical sense. Also, the Jordan River, Ganges River, and other bodies of water are considered sacred by people of certain religions.
Yet, water pollution, overconsumption, and uneven distribution have resulted in shortages of clean freshwater in many parts of the world. These shortages have in turn led to disputes between peoples of different nations.
Beyond the Earth, a significant quantity of water is thought to exist underground on the planet Mars, on Jupiter's moon Europa and Saturn's moon Enceladus, and also on exoplanets such as HD 189733 b[2] and HD 209458b.[3]

Chemical and physical properties
Water is a chemical compound with the chemical formula H2O. Each molecule of water consists of two hydrogen atoms covalently bonded to a single oxygen atom. At ambient temperature and pressure, water is a tasteless, odorless liquid. It appears colorless in small quantities, but it has an intrinsic very light blue hue. Pure ice also appears colorless, and water vapor is essentially invisible as a gas.[4]
Water is primarily a liquid under standard conditions—a property that makes it different from other analogous hydrides of the oxygen family in the periodic table. Those hydrides, such as hydrogen sulfide, are gases. Also, the elements surrounding oxygen in the periodic table—namely, nitrogen, fluorine, phosphorus, sulfur and chlorine—all combine with hydrogen to produce gases under standard conditions.
Polar nature of water molecules
Many of the properties of water can be explained by the polar nature of its molecules. The oxygen atom is strongly electronegative, and within each water molecule, the oxygen atom draws electrons closer to itself, away from the hydrogen atoms. As a result, there is a partial negative charge (δ-) near the oxygen atom and a partial positive charge (δ+) near each hydrogen atom. Thus the entire molecule is polar, with a net dipole moment. Due to this polarity, there is electrical attraction between water molecules, pulling them closer to one another. This attraction is called hydrogen bonding.
The hydrogen bonds between water molecules raise the boiling point of water and cause it to be a liquid at room temperature and pressure. By contrast, hydrogen sulfide is a gas under the same conditions because of the absence of such hydrogen bonds between its molecules.
Acids, bases, and pH values
Water is involved in common acid-base reactions. An acid (more precisely, a Brønsted-Lowry acid) is a donor of hydrogen ions (H+, or proton), and a base (Brønsted-Lowry base) is a hydrogen ion acceptor. When the base is a hydroxide ion (OH−), its reaction (neutralization) with an acid produces water (HOH).
Some water molecules react with one another to produce hydronium ions (H3O+(aq)) and hydroxide ions (OH−(aq)). In this case, one water molecule acts as an acid and donates a hydrogen ion to another, which acts as a base.
Water is also the usual standard for the measurement of pH—a quantity defined as the negative logarithm of the hydrogen ion concentration. When the pH of water (or a solution) is 7, it is said to be "neutral"—neither acidic nor basic. Acids (and acidic solutions) have pH values less than 7; bases (and basic solutions) have pH values greater than 7.
Cohesion and adhesion
Given the polar nature of water molecules, water tends to stick to itself—a property known as cohesion. At the same time, the polar nature of water molecules also explains the ability of water to stick to other surfaces—a property known as adhesion. For example, water may form a thin film on clean, smooth glass because the adhesive forces between glass and water molecules are stronger than the cohesive forces.
In biological cells, water tends to stick to hydrophilic (water-attracting) surfaces of proteins and membranes. To dehydrate hydrophilic surfaces—that is, to remove the strongly held layers of water—requires doing substantial work against these forces, called hydration forces. These forces are particularly important when cells are exposed to dry atmospheres or during extracellular freezing.
Surface tension

Water has a high surface tension caused by the strong cohesion between water molecules. This can be seen when small quantities of water are put onto a non-soluble surface such as polythene; the water stays together as drops. Just as significantly, air trapped in surface disturbances forms bubbles, which sometimes last long enough to transfer gas molecules to the water.
Another surface tension effect is capillary waves. These are the surface ripples that form from around the impact of drops on water surfaces, and sometimes occur when strong subsurface currents flow to the water surface. The apparent elasticity caused by surface tension drives the waves.
Capillary action
Capillary action refers to the process of water moving up a narrow tube against the force of gravity. It occurs because (a) water adheres to the sides of the tube; (b) surface tension tends to straighten the surface, making the surface rise; and (c) more water is pulled up through cohesion. The process is repeated as the water flows up the tube, until the water reaches a level where gravity counteracts the adhesive forces.
Solvation
Water is a very strong solvent and dissolves many types of substances. It has therefore been called the universal solvent. Substances that will mix well and dissolve in water (such as salts) are known as "hydrophilic" (water-loving) substances; those that do not mix well with water (such as fats and oils), are called "hydrophobic" (water-fearing) substances. The ability of a substance to dissolve in water is determined by whether or not the substance can match or better the strong attractive forces that water molecules generate among themselves. If the properties of a substance do not allow it to overcome these strong intermolecular forces, the molecules are "pushed out" from the water and do not dissolve.
Electrical conductivity
Pure water has low electrical conductivity, but it increases significantly upon solvation of even a small amount of ionizable material, such as hydrogen chloride. Thus the risks of electrocution are much greater in water with the usual impurities not found in pure water. Any electrical properties observable in water are from the ions of mineral salts and carbon dioxide dissolved in it.
Some molecules of water dissociate into ions, producing hydroxide anions and hydronium cations, as noted earlier. This dissociation is at a very low level in pure water, so the water will not carry enough electric current to do any work or cause any harm for most operations. In pure water, sensitive equipment can detect a very slight electrical conductivity of 0.055 µS/cm at 25 °C. Water can also be electrolyzed into oxygen and hydrogen gases, but in the absence of dissolved ions this is a very slow process, as very little current is conducted.
Water containing deuterium and tritium
Hydrogen has three isotopes. The most common isotope, present in more than 95 percent of water, has 1 proton and no neutron in the atomic nucleus. A second isotope, deuterium (or "D"), has 1 proton and 1 neutron. Water that contains deuterium (D2O) is also known as heavy water and is used in nuclear reactors for storing nuclear wastes. The third isotope, tritium (or "T"), has 1 proton and 2 neutrons in the atomic nucleus, and is radioactive. Water that contains tritium (T2O) does not exist in nature, as the creation of the molecule would result in its almost instantaneous decomposition. D2O is stable, but it differs from H2O in being denser. Also, it can block alpha and beta rays. D2O occurs naturally in water at very low concentrations. Consumption of pure isolated D2O adversely affects biochemical processes: ingestion of large amounts impairs kidney and central nervous system functions.
Heat capacity and heat of vaporization
Water has the second highest specific heat capacity of any known chemical compound, after ammonia. In addition, it has a high heat of vaporization (40.65 kJ mol−1). Both these properties are a result of the extensive hydrogen bonding between its molecules. These two unusual properties allow water to moderate Earth's climate by buffering large fluctuations in temperature.
Ice floats on liquid water
A simple but environmentally important and unusual property of water is that its solid form, ice, floats on its liquid form, because ice has a lower density than liquid water. By contrast, for almost all other substances, the solid form has a higher density than the liquid form. This property of water can be explained as follows.
When freshwater is cooled, it increases in density, and the cooler water sinks below the warmer layers by convection. This continues until the water reaches a temperature of 3.98 °C (at standard atmospheric pressure), at which stage water reaches its highest density. Further cooling lowers the density of water, because of the geometry of the hydrogen bonds formed between the molecules. When some of the water freezes, the ice that is formed floats because of its lower density.
When a body of water such as a lake begins to freeze, ice forms first at the surface and progresses downward. Water in the deeper regions of the lake remains warmer than that near the top. The layer of ice at the top effectively insulates the lake floor from the cold, protecting the fish and other living organisms from freezing to death.
Although water freezes at 0 °C (32 °F, 273 K), it can be supercooled in a fluid state down to its crystal homogeneous nucleation at almost 231 K (−42 °C)[5]. Ice also has a number of more exotic phases not commonly seen.
Triple point
| Phases in stable equilibrium | Pressure | Temperature |
|---|---|---|
| liquid water, ice I, and water vapour | 611.73 Pa | 273.16 K |
| liquid water, ice Ih, and ice III | 209.9 MPa | 251 K (-22 °C) |
| liquid water, ice Ih, and gaseous water | 612 Pa | 0.01 °C |
| liquid water, ice III, and ice V | 350.1 MPa | -17.0 °C |
| liquid water, ice V, and ice VI | 632.4 MPa | 0.16 °C |
| ice Ih, Ice II, and ice III | 213 MPa | -35 °C |
| ice II, ice III, and ice V | 344 MPa | -24 °C |
| ice II, ice V, and ice VI | 626 MPa | -70 °C |
The triple point of water is the combination of pressure and temperature at which pure liquid water, ice, and water vapor can coexist in a stable equilibrium. The phase diagram of water has several triple points, of which the most familiar one is used to define the kelvin (K), the SI unit of thermodynamic temperature. As a consequence, this triple point temperature is a prescribed value rather than a measured quantity: 273.16 K (0.01 °C) and a pressure of 611.73 pascals (approximately 0.0060373 atm). This triple point is approximately the combination that exists at 100 percent relative humidity at sea level and the freezing point of water.
Gustav Heinrich Johann Apollon Tammann in Göttingen produced data on several other triple points in the early twentieth century. Kamb and others documented further triple points in the 1960s.[7][6][8]
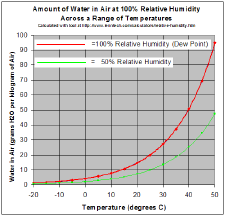
Miscibility, condensation, and relative humidity
Water is miscible with many liquids, for example ethanol in all proportions, forming a single homogeneous liquid. On the other hand water and most oils are immiscible usually forming layers according to increasing density from the top.
As a gas, water vapor is completely miscible with air. On the other hand the maximum water vapor pressure that is thermodynamically stable with the liquid (or solid) at a given temperature is relatively low compared with total atmospheric pressure. For example, if the vapor partial pressure[9] is 2 percent of atmospheric pressure and the air is cooled from 25 °C, starting at about 22 °C water will start to condense, defining the dew point, and creating fog or dew. The reverse process accounts for the fog burning off in the morning.
If one raises the humidity at room temperature, say by running a hot shower or a bath, and the temperature stays about the same, the vapor soon reaches the pressure for phase change, and condenses out as steam.
A gas in this context is referred to as saturated or 100 percent relative humidity, when the vapor pressure of water in the air is at the equilibrium with vapor pressure due to (liquid) water; water (or ice, if cool enough) will fail to lose mass through evaporation when exposed to saturated air. Because the amount of water vapor in air is small, relative humidity, the ratio of the partial pressure due to the water vapor to the saturated partial vapor pressure, is much more useful.
Water vapor pressure above 100 percent relative humidity is called super-saturated and can occur if air is rapidly cooled, say by rising suddenly in an updraft.[10]
Water on Earth
Water is found in a variety of locations on Earth, in solid, liquid, and gaseous states. Accordingly, it is known by different names: water vapor and clouds in the sky; seawater and icebergs in the ocean; glaciers and rivers in the mountains; and aquifers in the ground. About 1,460 teratonnes (Tt)[11] of water covers about 71 percent of the Earth's surface. Saltwater oceans hold 97 percent of surface water, glaciers and polar ice caps 2.4 percent, and other land surface water such as rivers and lakes 0.6 percent.
Origin and planetary effects
It is thought that much of the universe's water may have been produced as a by-product of star formation. The birth of a star is accompanied by a strong outward wind of gas and dust. When this outflow of material eventually impacts the surrounding gas, the resultant shock waves compress and heat the gas. Water could be quickly produced in this warm, dense gas.[12]
Earth's habitability
The existence of liquid water, and to a lesser extent its gaseous and solid forms, on Earth is vital to the existence of life on Earth. The Earth is located in the habitable zone of the Solar System. If it were slightly closer to or farther from the Sun (about 5 percent, or 8 million kilometers or so), the conditions that allow the three forms of water to be present simultaneously would be far less likely to prevail.[13][14]
Earth's mass allows its gravity to hold an atmosphere. Water vapor and carbon dioxide in the atmosphere provide a greenhouse effect that helps maintain a relatively steady surface temperature. If Earth were smaller, a thinner atmosphere would cause temperature extremes, preventing the accumulation of water except at the polar ice caps (as on Mars). If Earth were too massive, the water on it could have been in the solid state even at relatively high temperatures, because of the high pressure caused by gravity.
It has been proposed that life itself may maintain the conditions that have allowed its continued existence. Earth's surface temperature has been relatively constant through geologic time, despite varying levels of incoming solar radiation (insolation), indicating that a dynamic process governs Earth's temperature via a combination of greenhouse gases and surface or atmospheric albedo. This proposal is known as the Gaia hypothesis.
Tides
Tides are the cyclic rising and falling of Earth's ocean surface caused by the tidal forces of the Moon and the Sun acting on the oceans. Tides cause changes in the depth of the marine and estuarine water bodies and produce oscillating currents known as tidal streams. The changing tide produced at a given location is the result of the changing positions of the Moon and Sun relative to the Earth coupled with the effects of Earth rotation and the local bathymetry. The strip of seashore that is submerged at high tide and exposed at low tide, the intertidal zone, is an important ecological product of ocean tides.
Water cycle
The biosphere can be roughly divided into oceans, land, and atmosphere. Water moves perpetually through each of these regions in the water cycle, which consists of following transfer processes:
- evaporation from oceans and other water bodies into the air and transpiration from land plants and animals into air.
- precipitation, from water vapor condensing from the air and falling to earth or ocean.
- runoff from the land usually reaching the sea.

Most water vapor over the oceans returns to the oceans, but winds carry water vapor over land at the same rate as runoff into the sea, about 36 Tt per year. Over land, evaporation and transpiration contribute another 71 Tt per year. Precipitation, at a rate of 107 Tt per year over land, has several forms: most commonly rain, snow, and hail, with some contribution from fog and dew. Condensed water in the air may also refract sunlight to produce rainbows.
Water runoff often collects over watersheds flowing into rivers. Some of this is diverted to irrigation for agriculture. Rivers and seas offer opportunity for travel and commerce. Through erosion, runoff shapes the environment creating river valleys and deltas that provide rich soil and level ground for the establishment of population centers.
Freshwater storage
Some runoff water is trapped for periods, for example in lakes. In addition, snow and ice collect at the poles, on high mountains, and in other regions that experience cold winters. Water also infiltrates the ground and goes into aquifers. This groundwater later flows back to the surface in springs, or more spectacularly in hot springs and geysers. Groundwater may be extracted artificially by digging wells.
These forms of water storage are important because clean, freshwater is essential for human and other land-based life forms. In many parts of the world, freshwater is in short supply.
Tastes and odors of water
Given that water can dissolve many different substances, it acquires different tastes and odors. In fact, humans and animals have developed senses to be able to evaluate the potability of water. Animals generally dislike the taste of salty sea water and the putrid swamps and favor the purer water of a mountain spring or aquifer. The taste advertised in spring water or mineral water derives from the minerals dissolved in it, as pure H2O is tasteless. The "purity" of spring and mineral water refers to the absence of toxins, pollutants, and harmful microbes.
Effects on life
Water has many distinct properties that are critical for the proliferation of all known forms of life, setting it apart from other substances. It is vital both as a solvent in which many of the body's solutes dissolve and as an essential part of many metabolic processes within the body, including reactions that lead to cellular replication and growth.
Metabolism is the sum total of anabolism and catabolism. In anabolism, water is removed from molecules (through energy-requiring enzymatic reactions) to build larger molecules (such as starches, triglycerides, and proteins for storage of fuels and information). In catabolism, water is used to break bonds, to generate smaller molecules (such as glucose, fatty acids, and amino acids). Water is thus essential and central to these metabolic processes. Without water, these metabolic processes would cease to exist.
Biochemical reactions take place in water at specific pH values. For instance, human enzymes usually perform optimally around a pH of 7.4. Digestion of food in the stomach requires the activity of an acid (hydrochloric acid, HCl). Some people suffer from what is called "acid reflux," in which the stomach acid makes its way into and adversely affects the esophagus. This condition can be temporarily neutralized by ingestion of a base such as aluminum hydroxide to produce the neutral molecules of water and aluminum chloride (a salt).
Water is also central to photosynthesis and respiration. Photosynthetic cells use the Sun's energy to split off water's hydrogen from oxygen. Hydrogen is combined with carbon dioxide (absorbed from air or water) to form glucose and release oxygen. All living cells use such fuels and oxidize the hydrogen and carbon to capture the Sun's energy and reform water and carbon dioxide in the process (cellular respiration).
Aquatic life forms
Earth's waters are filled with life. Nearly all fish live exclusively in water, and many marine mammals, such as dolphins and whales, also live in the water. Some kinds of animals, such as amphibians, spend portions of their lives in water and portions on land. Plants such as kelp and algae grow in the water and are the basis for some underwater ecosystems. Plankton is generally the foundation of the ocean food chain.
Different water creatures use different ways of obtaining oxygen in the water. Fish have gills instead of lungs, although some species of fish, such as the lungfish, have both. Marine mammals, such as dolphins, whales, otters, and seals, need to surface periodically to breathe air.
Human uses
Civilization has historically flourished around rivers and major waterways. Mesopotamia, the so-called cradle of civilization, was situated between the major rivers Tigris and Euphrates; the ancient Egyptians depended greatly upon the Nile. Large metropolitan areas like Rotterdam, London, Montreal, Paris, New York City, Shanghai, Tokyo, Chicago, Mumbai, and Hong Kong owe their success in part to their easy accessibility via water and the resultant expansion of trade. Islands with safe water ports, like Singapore, have flourished for the same reason. In regions such as North Africa and the Middle East, where freshwater is relatively scarce, access to clean drinking water has been a major factor in human development.
Water fit for human consumption is called drinking water or potable water. Water that is not potable can be made potable by various methods, including: filtration, to remove particulate impurities; chemical or heat treatment, to kill bacteria; and distillation, to separate water from impurities by vaporization and condensation. It should be noted, however, that some solutes in potable water are acceptable and even desirable for taste enhancement and to provide needed electrolytes.
Water that is not fit for drinking but is not harmful if used for swimming or bathing is sometimes called "safe water" or "safe for bathing." Chlorine, a skin and mucous membrane irritant, is used to make water safe for bathing or drinking. Its use is highly technical and is usually monitored by government regulations (typically 1 part per million (ppm) for drinking water, and 1-2 ppm of chlorine not yet reacted with impurities for bathing water).
The single largest freshwater resource suitable for drinking is Lake Baikal in Siberia, which has a very low salt and calcium content and is very clean.
Drinking water
About 70 percent of the fat-free mass of the human body is made of water. To function properly, the body requires between one and seven liters of water per day to avoid dehydration; the precise amount depends on the level of activity, temperature, humidity, and other factors. Most of this is ingested through foods or beverages other than drinking straight water. It is not clear how much water intake is needed by healthy people.
For those who have healthy kidneys, it is rather difficult to drink too much water, but (especially in warm humid weather and while exercising) it is dangerous to drink too little. People can drink far more water than necessary while exercising, however, putting them at risk of water intoxication, which can be fatal. The "fact" that a person should consume eight glasses of water per day cannot be traced back to a scientific source.[15] There are other myths such as the effect of water on weight loss and constipation that have been dispelled.
Original recommendation for water intake in 1945 by the Food and Nutrition Board of the National Research Council read: "An ordinary standard for diverse persons is 1 milliliter for each calorie of food. Most of this quantity is contained in prepared foods."[16] The latest dietary reference intake report by the United States National Research Council in general recommended (including food sources): 2.7 liters of water total for women and 3.7 liters for men.[17] Specifically, pregnant and breastfeeding women need additional fluids to stay hydrated. According to the Institute of Medicine—who recommend that, on average, women consume 2.2 liters and men 3.0 liters—this is recommended to be 2.4 liters (approx. 9 cups) for pregnant women and 3 liters (approx. 12.5 cups) for breastfeeding women, since an especially large amount of fluid is lost during nursing.[18] Also noted is that, normally, about 20 percent of water intake comes from food, while the rest comes from drinking water and beverages (caffeinated included). Water is excreted from the body in multiple forms: through urine, feces, sweating, and exhalation of water vapor in the breath. With physical exertion and heat exposure, water loss will increase and daily fluid needs may increase as well.
Agriculture
In many developing nations, irrigation accounts for over 90 percent of water withdrawn from available sources for use. In England, where rain is abundant year round, water used for agriculture accounts for less than 1 percent of human usage. Yet even on the same continent, water used for irrigation in Spain, Portugal and Greece exceeds 70 percent of total usage.
Irrigation has been a key component of the "green revolution," which has enabled many developing countries to produce enough food to feed everyone. More water will be needed to produce more food for 3 billion more people. But increasing competition for water and inefficient irrigation practices could constrain future food production.
As a cleaning agent
Water is important for washing the human body and everyday items such as clothes, floors, cars, food, and pets.
Standard of measurement
On April 7, 1795, the gram was defined in France to be equal to "the absolute weight of a volume of pure water equal to a cube of one hundredth of a meter, and to the temperature of the melting ice." For practical purposes though, a metallic reference standard was required, one thousand times more massive, the kilogram. Work was therefore commissioned to determine precisely how massive one liter of water was. In spite of the fact that the decreed definition of the gram specified water at 0 °C—a highly stable temperature point—the scientists chose to redefine the standard and to perform their measurements at the most stable density point: the temperature at which water reaches maximum density, which was measured at the time as 4 °C.
As a thermal transfer agent
Boiling, steaming, and simmering are popular cooking methods that often require immersing food in water or its gaseous state, steam. Water is also used in industrial contexts as a coolant, and in almost all power-stations as a coolant and to drive steam turbines to generate electricity. In the nuclear industry, water can also be used as a neutron moderator.
Recreation
Humans use water for many recreational purposes, as well as for exercising and sports. Some of these include swimming, waterskiing, boating, fishing, and diving. In addition, some sports, like ice hockey and ice skating, are played on ice. Likewise, sports such as skiing or snowboarding require the water to be frozen. Many use water for play fighting, such as with snowballs, water guns, or water balloons.
Lakesides and beaches are popular places for people to go for recreation and relaxation. Many find the sound of flowing water to be calming. Some keep fish and other life in water tanks or ponds for show, fun, and companionship. People also make fountains and use water in their public or private decorations.
Industrial applications
Pressurized water is used in water blasting and water jet cutters. Also, high-pressure water guns are used for precise cutting. It is also an effective coolant for various machines that generate heat during operation. It works very well, is relatively safe, and is not harmful to the environment.
Food processing
Water plays many critical roles within the field of food science. Food scientists need to understand the roles of water in food processing, to ensure the success of their products.
Solutes such as salts and sugars found in water affect the physical properties of water. The boiling and freezing points of water is affected by solutes. One mole of sucrose (sugar) raises the boiling point of water by 0.52 °C, and one mole of salt raises the boiling point by 1.04 °C while lowering the freezing point of water in a similar way.[19] Solutes in water also affect water activity which affects many chemical reactions and the growth of microbes in food.[20] Water activity can be described as a ratio of the vapor pressure of water in a solution to the vapor pressure of pure water.[19] Solutes in water lower water activity. This is important to know because most bacterial growth ceases at low levels of water activity.[20] Not only does microbial growth affect the safety of food but also the preservation and shelf life of food.
Water hardness is also a critical factor in food processing. It can dramatically affect the quality of a product as well as playing a role in sanitation. Water hardness is classified based on the amounts of removable calcium carbonate salt it contains per gallon. Water hardness is measured in grains; 0.064 g calcium carbonate is equivalent to one grain of hardness.[19] Water is classified as soft if it contains 1 to 4 grains, medium if it contains 5 to 10 grains and hard if it contains 11 to 20 grains.[19] The hardness of water may be altered or treated by using a chemical ion exchange system. The hardness of water also affects its pH balance which plays a critical role in food processing. For example, hard water prevents successful production of clear beverages. Water hardness also affects sanitation; with increasing hardness, there is a loss of effectiveness for its use as a sanitizer.[19]
Power generation
Hydroelectricity is electricity obtained from hydropower. Hydroelectric power comes from water driving a turbine connected to a generator. Hydroelectricity is a low-cost, non-polluting, renewable energy source.
Water resource distribution and pollution
Water in itself is not a finite resource (like petroleum is). The water cycle, which involves evaporation, condensation, and precipitation, regenerates potable water in large quantities, many orders of magnitude higher than human consumption. However, many parts of the world are experiencing water scarcity, in the sense that there are problems with the distribution of potable and irrigation water. Such shortages of water form a major social and economic concern and have led to disputes between nations that rely on the same source of water (such as the same river). Some countries experiencing water shortages import water or purify seawater by desalination.
Currently, about 1 billion people around the world routinely drink unhealthy water. Poor water quality and bad sanitation are deadly; some 5 million deaths a year are caused by polluted drinking water.
In the developing world, 90 percent of all wastewater goes untreated into local rivers and streams. Some 50 countries, with roughly a third of the world’s population, also suffer from medium or high water stress, and a number of them extract more water annually than is recharged through their natural water cycles. The strain affects surface freshwater bodies like rivers and lakes, but it also degrades groundwater resources.
Water is a strategic resource in the globe and an important element in many political conflicts. Some have predicted that clean water will become the "next oil," making Canada, with this resource in abundance, possibly the richest country in the world. There is a long history of conflict over water, including efforts to gain access to water, the use of water in wars started for other reasons, and tensions over shortages and control.[21]
UNESCO's World Water Development Report (WWDR, 2003) from its World Water Assessment Program indicates that, in the next 20 years, the quantity of water available to everyone is predicted to decrease by 30 percent. About 40 percent of the world's inhabitants currently have insufficient fresh water for minimal hygiene. More than 2.2 million people died in 2000 from diseases related to the consumption of contaminated water or drought. In 2004, the UK charity WaterAid reported that a child dies every 15 seconds from easily preventable water-related diseases; often this means lack of sewage disposal; see toilet.
Water availability in specific regions
Ninety-five percent of freshwater in the United States is underground. One crucial source is a huge underground reservoir, the 1,300-kilometer (800 mi) Ogallala aquifer which stretches from Texas to South Dakota and waters one fifth of U.S. irrigated land. Formed over millions of years, the Ogallala aquifer has since been cut off from its original natural sources. It is being depleted at a rate of 12 billion cubic meters (420 billion ft3) per year, amounting to a total depletion to date of a volume equal to the annual flow of 18 Colorado Rivers. Some estimates say it will dry up in as little as 25 years. Many farmers in the Texas High Plains, which rely particularly on the underground source, are now turning away from irrigated agriculture as they become aware of the hazards of overpumping.[22]
The Middle East region has only 1 percent of the world's available freshwater, which is shared among 5 percent of the world's population. Thus, in this region, water is an important strategic resource. It is predicted that by 2025, countries of the Arabian peninsula will be using more than twice the amount of water naturally available to them.[23] According to a report by the Arab League, two-thirds of Arab countries have less than 1,000 cubic meters (35,000 ft3) of water per person per year available, which is considered the limit.[24]
In Asia, Cambodia and Vietnam are concerned about attempts by China and Laos to control the flux of water. China is preparing the Three Gorges Dam project on the Yangtze River, which would become the world's largest dam, causing many social and environmental problems. It also has a project to divert water from the Yangtze to the dwindling Yellow River, which feeds China's most important farming region.
The Ganges is disputed between India and Bangladesh. The water reserves are being quickly depleted and polluted, while the glacier feeding the sacred Hindu river is retreating hundreds of feet each year, causing subsoil streams flowing into the Ganges river to dry up.
In South America, the Guaraní Aquifer is located between the Mercosur countries of Argentina, Brazil, Bolivia and Paraguay. With a volume of about 40,000 km³, it is an important source of fresh potable water for all four countries.
Purification and waste reduction
Drinking water is often collected at springs, extracted from artificial borings in the ground, or wells. Building more wells in adequate places is thus a possible way to produce more water, assuming the aquifers can supply an adequate flow. Other water sources are rainwater and river or lake water. This surface water, however, must be purified for human consumption. This may involve removal of undissolved substances, dissolved substances and harmful microbes. Popular methods are filtering with sand which only removes undissolved material, while chlorination and boiling kill harmful microbes. Distillation does all three functions. More advanced techniques are also available, such as reverse osmosis. Desalination of seawater is a more expensive solution, but it is used in some coastal areas with arid climates because the water is abundantly available.
The distribution of drinking water is done through municipal water systems or as bottled water. Governments in many countries have programs to distribute water to the needy at no charge. Others argue that the market mechanism and free enterprise are best to manage this rare resource and to finance the boring of wells or the construction of dams and reservoirs.
Reducing waste by using drinking water only for human consumption is another option. In some cities such as Hong Kong, seawater is extensively used for flushing toilets to conserve freshwater resources.
Polluting water may be the biggest single misuse of water; to the extent that a pollutant limits other uses of the water, it becomes a waste of the resource, regardless of benefits to the polluter. Like other types of pollution, this does not enter standard accounting of market costs, being conceived as externalities for which the market cannot account. Thus other people pay the price of water pollution, while the private firms' profits are not redistributed to the local people who are victims to this pollution. Pharmaceuticals consumed by humans often end up in the waterways and can have detrimental effects on aquatic life if they bioaccumulate.
Religion and philosophy
In most religions, water is considered a purifier in an internal, spiritual sense as well as in an external, physical sense. Faiths that incorporate ritual washing (ablution) include Hinduism, Christianity, Islam, Judaism, Zoroastrianism, and Shinto. Water is mentioned in the Bible 442 times in the New International Version and 363 times in the King James Version. For example, 2 Peter 3:5(b) states, "The earth was formed out of water and by water" (NIV).
Water baptism is a central sacrament of Christianity. It is also a part of the practice of other religions, including Judaism (mikvah) and Sikhism (Amrit Sanskar). In Zoroastrianism, one is expected to wash one's hands and face before praying in the fire temple. Likewise, in Islam, the five daily prayers can be offered in most cases after washing certain parts of the body with clean water (wudu). In Shinto, water is used in almost all rituals to cleanse a person or area (such as in the ritual of misogi). In addition, a ritual bath in pure water is performed for the dead in many religions, including Judaism and Islam.
Some faiths use water especially prepared for religious purposes—holy water in some Christian denominations; Amrit in Sikhism and Hinduism. Many religions also consider particular sources or bodies of water to be sacred or at least auspicious. Examples include Lourdes in Roman Catholicism, the Zamzam Well in Islam, and the River Ganges (among many others) in Hinduism. In Neo-Paganism water is often combined with salt in the first steps of a ritual, to act as a purifier of worshippers and the altar, symbolizing both cleansing tears and the ocean.
Water is often believed to have spiritual powers. In Celtic mythology, Sulis is the local goddess of thermal springs; in Hinduism, the Ganges is also personified as a goddess, while Saraswati has been referred to as a goddess in Vedas. Also water is one of the "panch-tatva"s (basic 5 elements, others including fire, earth, space, air).
Alternatively, gods can be patrons of particular springs, rivers, or lakes. For example, in Greek and Roman mythology, Peneus was a river god, one of the three thousand Oceanids. In Islam, not only does water give life, but every life is itself made of water: "We made from water every living thing".[25]
The Greek philosopher Empedocles held that water is one of the four classical elements along with fire, earth and air, and was regarded as the ylem, or basic substance of the universe. Water was considered cold and moist. In the theory of the four bodily humors, water was associated with phlegm. Water was also one of the five elements in traditional Chinese philosophy, along with earth, fire, wood, and metal.
Notes
- ↑ Laurence D. Barron, Lutz Hecht, and Gary Wilson, The Lubricant of Life: A Proposal That Solvent Water Promotes Extremely Fast Conformational Fluctuations in Mobile Heteropolypeptide Structure ACS Publications, 1997. Retrieved January 9, 2020.
- ↑ Pallab Ghosh, Water found for first time on 'potentially habitable' planet BBC News, September 12, 2019. Retrieved January 9, 2020.
- ↑ Ker Than, Water Found in Extrasolar Planet's Atmosphere. Space.com, April 10, 2007. Retrieved January 9, 2020.
- ↑ Charles L. Braun, Sergei N. Smirnov, Why is water blue? J. Chem. Educ. 70(8) (1993):612.
- ↑ P.G. Debenedetti, and H.E. Stanley, Supercooled and Glassy Water. Physics Today 56(6) (2003):40–46. Retrieved January 9, 2020.
- ↑ 6.0 6.1 Oliver Schlüter, Impact of High Pressure — Low Temperature Processes on Cellular Materials Related to Foods. Technischen Universität Berlin, 2003. Retrieved January 9, 2020.
- ↑ Gustav Tammann The States Of Aggregation. (Constable And Company Limited, 1925).
- ↑ William Cudmore McCullagh Lewis, and James Rice, A System of Physical Chemistry. (London, UK: Longmans, Green and co., 1922).
- ↑ The pressure due to water vapor in the air is called the partial pressure(Dalton's law) and it is directly proportional to concentration of water molecules in air (Boyle's law).
- ↑ Adiabatic cooling resulting from the ideal gas law.
- ↑ One tonne (or metric ton) is defined as 1000 kilograms (kg) or 1 megagram (Mg). One teratonne is equal to 1012 tonnes.
- ↑ Gary Melnick and David Neufeld, Space Cloud Holds Enough Water to Fill Earth's Oceans 1 Million Times, Headlines@Hopkins, JHU, 1998. Retrieved January 9, 2020.
- ↑ E. Ehlers and T. Krafft (eds.), Understanding the Earth System: compartments, processes, and interactions. (New York, NY: Springer, 2001).
- ↑ Habitable Zone. The Encyclopedia of Astrobiology, Astronomy and Spaceflight. Retrieved January 9, 2020.
- ↑ Heinz Valdin, "Drink at least eight glasses of water a day." Really? Is there scientific evidence for "8 × 8"? Department of Physiology, Dartmouth Medical School. Retrieved January 9, 2020.
- ↑ Food and Nutrition Board, National Academy of Sciences. Recommended Dietary Allowances, revised 1945. National Research Council, Reprint and Circular Series. 122:3-18.
- ↑ Dietary Reference Intakes: Water, Potassium, Sodium, Chloride, and Sulfate. Food and Nutrition Board, National Institute of Medicine, 2005. Retrieved January 9, 2020.
- ↑ Water: How much should you drink every day? Mayo Clinic. Retrieved January 9, 2020.
- ↑ 19.0 19.1 19.2 19.3 19.4 Vickie A. Vaclavik and Elizabeth W. Christian, Essentials of Food Science (New York, NY: Kluwer Academic/Plenum Publishers, 2003, ISBN 0306473631).
- ↑ 20.0 20.1 John M. DeMan, Principles of Food Chemistry (Gaithersburg, MD: Aspen Publishers, 1999, ISBN 083421234X).
- ↑ A Chronology of Water-Related Conflicts The World's Water, Pacific Institute. Retrieved January 9, 2020.
- ↑ Ogallala aquifer - Water hot spots. BBC News. Retrieved January 9, 2020.
- ↑ Ben Sutherland, Water shortages 'foster terrorism' BBC News, March 18, 2003. Retrieved January 9, 2020.
- ↑ Christian Chesnot, "Major aspects of scarce water resources management with reference to the Arab countries," Arab League report published for the International Conference on water gestion and water politics in arid zones, in Amman, Jordan, December 1-3, 1999. in Drought in the Middle East. (Monde diplomatique.)
- ↑ Sura of Al-Anbiya 21:30
ReferencesISBN links support NWE through referral fees
- Anderson, Terry L. Water Rights: Scarce Resource Allocation, Bureaucracy, and the Environment. Cambridge, MA: Ballinger Pub. Co., 1991. ISBN 0884103900.
- Barlow, Maude, Tony Clarke. Blue Gold: The Fight to Stop the Corporate Theft of the World's Water. New York, NY: New Press (distributed by W.W. Norton), 2003. ISBN 1565847318.
- de Villiers, Marq. Water: The Fate of Our Most Precious Resource. Toronto, ON: M&S, 2003. ISBN 0771026412.
- Debenedetti, P.G., and H.E. Stanley. Supercooled and Glassy Water. Physics Today 56(6) (2003):40–46. Retrieved January 9, 2020.
- DeMan, John M. Principles of Food Chemistry 3rd Edition. Gaithersburg, MD: Aspen Publishers, 1999. ISBN 083421234X.
- Ehlers, Eckart, and T. Krafft (eds.). Understanding the Earth System: compartments, processes, and interactions. New York, NY: Springer,2001. ISBN 3540675159.
- Franks, F. (ed.). Water, A comprehensive treatise. New York, NY: Plenum Press, 1982. ISBN 0306407108.
- Gleick, Peter H. The World's Water: The Biennial Report on Freshwater Resources. Washington, DC: Island Press, 2006. ISBN 978-1597261050.
- Jones, O.A., J.N. Lester and N. Voulvoulis. Pharmaceuticals: a threat to drinking water? TRENDS in Biotechnology 23(4) (2005):163.
- Lowi, Miriam R. Water and Power: The Politics of a Scarce Resource in the Jordan River Basin. New York, NY: Cambridge University Press, 1995. ISBN 0521431646.
- Marks, William E. The Holy Order of Water: Healing Earth's Waters and Ourselves. Great Barrington, MA: Bell Pond Books (div. of Steiner Books), 2001. ISBN 088010483X
- Postel, Sandra. Last Oasis: Facing Water Scarcity. New York, NY: Norton Press, 1997. ISBN 0393317447.
- Reisner, Marc. Cadillac Desert: The American West and Its Disappearing Water. New York, NY: Penguin Books, 1993. ISBN 0140178244.
- Roddick, Anita et al. Troubled Water: Saints, Sinners, Truth And Lies About The Global Water Crisis. White River Jct., VT: Chelsea Green Publishing Company, 2004. ISBN 095439593X.
- Shiva, Vandana. Water Wars: Privatization, Pollution, and Profit. Cambridge, MA: South End Press, 2002. ISBN 0745318371.
- Vaclavik, Vickie A. and Elizabeth W. Christian. Essentials of Food Science, 2nd Edition. New York, NY: Kluwer Academic/Plenum Publishers, 2003. ISBN 0306473631.
- Ward, Diane Raines. Water Wars: Drought, Flood, Folly and the Politics of Thirst. New York. NY: Riverhead Books, 2002. ISBN 1573222291.
- Worster, Donald. Rivers of Empire: Water, Aridity, and the Growth of the American West. New York, NY: Pantheon Books, 1992. ISBN 039451680X.
External links
All links retrieved May 3, 2023.
- American Water Resources Association.
- WaterAid Charity dedicated to the provision of clean water, sanitation and hygiene education.
- Water and Steam Tables for Industrial Use.
| Food chemistry |
|---|
| Carbohydrates • Colors • Enzymes • Fatty acids * Flavors • Food additives • Lipids • Minerals • Proteins • Vitamins • Water |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.