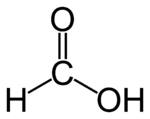
Formic acid
| Formic acid | |
|---|---|
| |
| General | |
| Systematic name | Methanoic acid |
| Other names | Hydrogen carboxylic acid Formylic acid Aminic acid |
| Molecular formula | CH2O2 HCOOH |
| SMILES | O=CO |
| Molar mass | 46.0254 g/mol |
| Appearance | Colorless, fuming liquid |
| CAS number | [64-18-6] |
| Properties | |
| Density and phase | 1.22 g/mL, liquid |
| Solubility in water | Miscible |
| Other solvents | Ethanol, acetone, ether |
| Melting point | 8.4°C (47.1°F) |
| Boiling point | 100.8°C (213.3°F) |
| Acidity (pKa) | 3.75 |
| Viscosity | 1.57 cP at 26°C |
| Structure | |
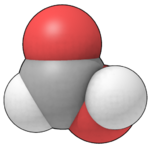
| Molecular shape | Planar |
| Dipole moment | 1.41 D(gas) |
| Hazards | |
| MSDS | ScienceLab.com |
| Main hazards | Corrosive; irritant; sensitizer. |
| NFPA 704 | |
| Flash point | 69°C (156°F) |
| R-phrases | R10, R35 |
| S-phrases | S1/2, S23, S26, S45 |
| RTECS number | LQ4900000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related carboxylic acids | Acetic acid Propionic acid |
| Related compounds | Formaldehyde Methanol |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) | |
Formic acid (systematic name methanoic acid) is the simplest carboxylic acid. Its formula is HCOOH or CH2O2. In nature, it is found in the stings and bites of many insects of the order Hymenoptera, particularly ants. Currently, it is used as a preservative in livestock feed, as an intermediate in chemical synthetic processes, and as the active ingredient in some household limescale removers. It is also a significant combustion product released by vehicles running on methanol mixed with gasoline. A salt or an ester of formic acid is called a formate or methanoate. The formate ion has the formula HCOO−.
This acid needs to be handled with care. Contact with liquid formic acid or its concentrated vapors can irritate and damage the skin, eyes, and respiratory tract.
History and etymology
As early as the fifteenth century, some alchemists and naturalists were aware that ant hills gave off an acidic vapor. In 1671, English naturalist John Ray became the first person to describe the isolation of formic acid by the distillation of large numbers of ants. These insects secrete the substance for attack and defense purposes. Thus the name "formic acid" was coined from the Latin word for ant, formica.
Formic acid was first synthesized from hydrocyanic acid by French chemist Joseph Gay-Lussac. In 1855, another French chemist, Marcellin Berthelot, developed a synthesis from carbon monoxide, a method similar to the one used today.
In the chemical industry, formic acid was long considered a compound of minor interest. In the late 1960s, however, significant quantities of it became available as a byproduct of acetic acid production. It is now increasingly used as a preservative and antibacterial in livestock feed.
Properties
Formic acid is miscible with water and most polar organic solvents, and somewhat soluble in hydrocarbons. Most simple formate salts are soluble in water.
When dissolved in hydrocarbons and when in the vapor phase, formic acid consists of hydrogen-bonded dimers (pairs of molecules) rather than individual molecules. In the gas phase, this hydrogen-bonding results in severe deviations from the ideal gas law. In the liquid and solid states, this acid consists of a network of hydrogen-bonded molecules. When heated, formic acid decomposes to carbon monoxide and water.
Formic acid shares most of the chemical properties of other carboxylic acids, but it also displays several unique properties. For instance, under normal conditions, it will not form either an acyl chloride or an acid anhydride. Until very recently, all attempts to form either of these derivatives resulted in carbon monoxide instead. It has now been shown that the anhydride may be produced by reaction of formyl fluoride with sodium formate at −78°C. The chloride can be produced by passing HCl into a solution of 1-formimidazole in monochloromethane at −60°C[1]. In addition, formic acid shares some of the reducing properties of aldehydes.
Formic acid is unique among the carboxylic acids in its ability to participate in addition reactions with alkenes, producing formate esters. In the presence of certain acids, including sulfuric acid and hydrofluoric acid, however, another reaction (a variant of the Koch reaction) takes place, in which formic acid adds to the alkene to produce a larger carboxylic acid.
Production
A significant amount of formic acid is obtained as a byproduct in the manufacture of other chemicals, especially acetic acid. As this production route is insufficient to meet the present demand, some formic acid must be produced for its own sake.
When methanol and carbon monoxide are combined in the presence of a strong base, the product is methyl formate, an ester of formic acid. The chemical equation may be written as:
In industry, this reaction is performed in the liquid phase at elevated pressure. Typical reaction conditions are 80°C and 40 atmospheres (atm) pressure. The most widely used base is sodium methoxide. Hydrolysis of the methyl formate produces formic acid:
Direct hydrolysis of methyl formate requires a large excess of water to proceed efficiently, and some producers use an indirect route.
In the laboratory, formic acid can be obtained by heating oxalic acid in anhydrous glycerol, extracting the product by steam distillation. Another preparation (which must be performed under a fume hood) is the acid hydrolysis of ethyl isonitrile using hydrochloric acid solution.[1][2]
Uses
The principal use of formic acid is as a preservative and antibacterial agent in livestock feed. When sprayed on fresh hay or other silage, it arrests certain decay processes and causes the feed to retain its nutritive value longer, and so it is widely used to preserve winter feed for cattle. In the poultry industry, it is sometimes added to feed to kill Salmonella bacteria.
Additional uses:
- It is used to process organic latex (sap) into raw rubber.
- Beekeepers use formic acid as a miticide against the Varroa mite.
- It is of minor importance in the textile industry and for the tanning of leather.
- Some formate esters are artificial flavorings or perfumes.
- It is the active ingredient in some brands of household limescale remover.
- It is used in laboratories as a solvent modifier for HPLC separations of proteins and peptides, especially when the sample is being prepared for mass spectrometry analysis.
- In synthetic organic chemistry, formic acid is often used as a source of the hydride ion (by the Eschweiler-Clarke reaction or the Leuckart-Wallach reaction) and as a source of hydrogen in what is called "transfer hydrogenation."
- In the laboratory, formic acid is used as a source of carbon monoxide, which is released by the addition of sulfuric acid. It is also a source for a formyl group in a reaction known as "formylation."[3]
Safety
The principal danger from formic acid is from skin or eye contact with liquid formic acid or with the concentrated vapors. Any of these exposure routes can cause severe chemical burns, and eye exposure can result in permanent eye damage. Inhaled vapors may similarly cause irritation or burns in the respiratory tract. Since carbon monoxide may also be present in formic acid vapors, care should be taken wherever large quantities of formic acid fumes are present. The U.S. OSHA Permissible Exposure Level (PEL) of formic acid vapor in the work environment is five parts per million parts of air (ppm).
Formic acid is readily metabolized and eliminated by the body. Nonetheless, some chronic effects have been documented. Some animal experiments have demonstrated it to be a mutagen, and chronic exposure may cause liver or kidney damage. Another possibility with chronic exposure is development of a skin allergy that manifests upon re-exposure to the chemical.
The hazards of solutions of formic acid depend on the concentration. The following table lists the EU classification of formic acid solutions:
| Concentration by weight |
Classification | R-Phrases |
|---|---|---|
| two to ten percent | Irritant (Xi) | R36/38 |
| 10–90 percent | Corrosive (C) | R34 |
| >90 percent | Corrosive (C) | R35 |
See also
Notes
- ↑ Julius B. Cohen, Practical Organic Chemistry (MacMillan, 1930).
- ↑ The isonitrile is obtained by reacting ethyl amine with chloroform. The fume hood is required because of the overpoweringly objectionable odor of the isonitrile.
- ↑ L.F. Fieser and J.E. Jones, "N-methylformanilide" Organic Syntheses Coll. Vol. 3 (1955): 590; 20(1940): 66. Retrieved December 31, 2007.
ReferencesISBN links support NWE through referral fees
- Bloch, D. R. Organic Chemistry Demystified. New York: McGraw-Hill, 2006. ISBN 0071459200.
- Cohen, Julius B. Practical Organic Chemistry. MacMillan, 1930.
- McMurry, John. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0136436692.
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998.
External links
All links retrieved April 1, 2024.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.