Formaldehyde
| Formaldehyde | |
|---|---|
| |
| General | |
| Common name | formaldehyde |
| IUPAC name | formaldehyde |
| Systematic name | methanal |
| Other names | formalin, formol, methyl aldehyde, methylene oxide |
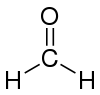
| Molecular formula | CH2O |
| SMILES | C=O |
| Molar mass | 30.03 g·mol−1 |
| Appearance | colourless gas |
| CAS number | [50-00-0] |
| Properties | |
| Density and phase | 1 kg·m−3, gas |
| Solubility in water | > 100 g/100 ml (20 °C) |
| in ethanol, acetone, DMSO |
> 100 g/100 ml |
| in ether, benzene, organic solvents |
soluble |
| in chloroform | immiscible |
| Melting point | -117 °C (156 K) |
| Boiling point | -19.3 °C (253.9 K) |
| Vapor pressure | 3890 mm Hg at 25 °C |
| Structure | |
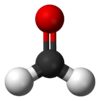
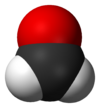
| Molecular shape | trigonal planar |
| Dipole moment | 2.33168(1) D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | toxic, flammable |
| NFPA 704 | |
| Flash point | -53 °C |
| R/S statement | R23/24/25, R34, R40, R43 S1/2, S26, S36/37, S39, S45, S51 |
| RTECS number | LP8925000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related aldehydes | acetaldehyde benzaldehyde |
| Related compounds | ketones carboxylic acids |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) | |
The chemical compound formaldehyde (also known as methanal) is a gas with a pungent smell. It is the simplest aldehyde. Its chemical formula is H2CO. Formaldehyde was first synthesized by the Russian chemist Aleksandr Butlerov in 1859 but was conclusively identified by August Wilhelm von Hofmann in 1868.[1]
Formaldehyde readily results from the incomplete combustion of carbon-containing materials. It may be found in the smoke from forest fires, in automobile exhaust, and in tobacco smoke. In the atmosphere, formaldehyde is produced by the action of sunlight and oxygen on atmospheric methane and other hydrocarbons. Small amounts of formaldehyde are produced as a metabolic byproduct in most organisms, including humans.
It is mainly produced by the oxidation of methanol, itself obtained from natural gas.
Properties
Although formaldehyde is a gas at room temperature, it is readily soluble in water. It is most commonly sold as a 37 percent aqueous solution with trade names such as formalin or formol. In water, formaldehyde converts to the hydrate CH2(OH)2. Thus formalin contains very little H2CO. These solutions usually contain a few percent methanol to limit the extent of polymerization.
Formaldehyde exhibits most of the chemical properties of the aldehydes, except that it is more reactive. Formaldehyde is a good electrophile. It can participate in electrophilic aromatic substitution reactions with aromatic compounds and can undergo electrophilic addition reactions with alkenes. In the presence of basic catalysts, formaldehyde undergoes a Cannizaro reaction to produce formic acid and methanol. Formalin reversibly polymerizes to produce its cyclic trimer, 1,3,5-trioxane or the linear polymer polyoxymethylene. Because of the formation of these derivatives, formaldehyde gas deviates strongly from the ideal gas law, especially at high pressure or low temperature.
Formaldehyde is readily oxidized by atmospheric oxygen to form formic acid. Formaldehyde solutions should be protected from air.
Production of Formaldehyde
Industrially, formaldehyde is produced by the catalytic oxidation of methanol. The most commonly used catalysts are silver metal or a mixture of an iron oxide with molybdenum and vanadium. In the more commonly used FORMOX® process (FORMOX® is a worldwide registered trademark owned by Perstorp Specialty Chemicals AB, Sweden - home page www.perstorp.com) methanol and oxygen react at ca 250-400°C in presence of iron oxide in combination with molybdenium and/or vanadium to produce formaldehyde according to the chemical equation
The silver-based catalyst is usually operated at a higher temperature, about 650 °C. On it, two chemical reactions simultaneously produce formaldehyde: the one shown above, and the dehydrogenation reaction
Further oxidation of the formaldehyde product during its production usually gives formic acid that is found in formaldehyde solution, found in ppm values.
On a smaller scale, formalin can be produced using a whole range of other methods including conversion from ethanol instead of the normally-fed methanol feedstock. Such methods are of less commercial importance.
Biology
An aqueous solution of formaldehyde can be used as a disinfectant as it kills most bacteria and fungi (including their spores). It is also used as a preservative in vaccinations. In medicine, formaldehyde solutions are applied topically to dry the skin, such as in the treatment of warts.
Formaldehyde is usually sold as a saturated aqueous solution with concentration of around 37 percent formaldehyde, stabilized with 10-15 percent methanol. The commercial name is either Formalin, or Formol. Formaldehyde preserves or fixes tissue or cells by irreversibly cross-linking primary amine groups in proteins with other nearby nitrogen atoms in protein or DNA through a -CH2- linkage.
Formaldehyde based solutions are used in embalming to disinfect and temporarily preserve human remains pending final disposition. It is the ability of formaldehyde to fix the tissue that produces the tell-tale firmness of flesh in an embalmed body. While other, heavier aldehydes also produce a similar firming action, none approaches the completeness of formaldehyde.
Formaldehyde is also used as a detergent in RNA gel electrophoresis, preventing RNA from forming secondary structures.
Industry
Most formaldehyde is used in the production of polymers and other chemicals. When combined with phenol, urea, or melamine, formaldehyde produces a hard thermoset resin. These resins are commonly used in permanent adhesives, such as those used in plywood or carpeting. It is used as the wet-strength resin added to sanitary paper products such as (listed in increasing concentrations injected into the paper machine headstock chest) facial tissue, table napkins, and roll towels. They are also foamed to make insulation, or cast into moulded products. Production of formaldehyde resins accounts for more than half of formaldehyde consumption.
Formaldehyde is still used in low concentrations for process C-41 (color negative film) stabilizer in the final wash step, as well as in the process E-6 pre-bleach step, to obviate the need for it in the final wash.
Formaldehyde is also used to make numerous other chemicals, used in personal care products such as toothpaste. Many of these are polyfunctional alcohols such as pentaerythritol, which is used to make paints and explosives. Other formaldehyde derivatives include methylene diphenyl diisocyanate, an important component in polyurethane paints and foams, and hexamine, which is used in phenol-formaldehyde resins and to make the explosive RDX.
Formaldehyde, along with 18 M (concentrated) sulfuric acid (the entire solution often called the Marquis reagent)[2] is used as an MDMA "testing kit." The solution alone cannot verify the presence of MDMA, but reacts with many other chemicals that the MDMA tablet itself may be adulterated with. The reaction itself produces colors which correlate with such chemicals.
Uses
Formaldehyde is primarily used to produce glues used in the manufacture of particleboard, veneers, wood furniture and other wood products. Formaldehyde is also used in the manufacture of various plastics, some fertilizers, resins used in foundry sand moulds, and some paints and varnishes. The textile industry uses these resins as finishers to make fabrics crease-resistant. The substance is also used in the synthesis of other chemical products and for its bactericidal properties in many formulations of disinfectant products, cosmetics, embalming fluids and solutions for preserving biological tissues.
Health effects
High amounts of formaldehyde can be toxic. Because formaldehyde resins are used in many construction materials, including plywood and spray-on insulating foams, and because these resins slowly give off formaldehyde over time, formaldehyde is one of the more common indoor air pollutants. At concentrations above 0.1 ppm in air, formaldehyde can irritate the eyes and mucous membranes, resulting in watery eyes. If inhaled, formaldehyde at this concentration may cause headaches, a burning sensation in the throat, and difficulty breathing.[3] The United States Environmental Protection Agency USEPA allows no more than 0.016 ppm formaldehyde in the air in new buildings constructed for that agency[4]
Large formaldehyde exposures, for example from drinking formaldehyde solutions, are potentially deadly. Formaldehyde is converted to formic acid in the body, leading to a rise in blood acidity (acidosis), rapid, shallow breathing, blurred vision or complete blindness, hypothermia, and, in the most severe cases, coma or death. People who have ingested formaldehyde require immediate medical attention.
In the body, formaldehyde can cause proteins to irreversibly bind to DNA. Laboratory animals exposed to large doses of inhaled formaldehyde over their lifetimes have developed more cancers of the nose and throat than are usual, as have workers in particle-board sawmills. However, some studies suggest that smaller concentrations of formaldehyde like those encountered in most buildings have no carcinogenic effects. Formaldehyde is classified as a probable human carcinogen by the U.S. Environmental Protection Agency, and as having sufficient evidence that formaldehyde causes nasopharyngeal cancer in humans by the International Agency for Research on Cancer.[5] Several European countries restrict the use of formaldehyde, including the import of formaldehyde-treated products and embalming, and the European Union is considering a complete ban on formaldehyde usage (including embalming), subject to a review of List 4B of the Technical Annex to the Report from the Commission to the European Parliament and the Council on the Evaluation of the Active Substances of Plant Protection Products by the European Commission Services. Countries with a strong tradition of embalming corpses, such as Ireland and other colder weather countries, have raised concerns.
Formaldehyde can cause allergies, and is part of the standard patch test series. People with formaldehyde allergy are advised to avoid formaldehyde-releasing chemicals as well (e.g., Quaternium-15, imidazolidinyl urea, and diazolidinyl urea).[6]
Occupational Health and Safety
Occupational exposure to formaldehyde by inhalation is mainly from three types of sources: thermal or chemical decomposition of formaldehyde-based resins, formaldehyde emission from aqueous solutions (for example, embalming fluids), or the production of formaldehyde resulting from the combustion of a variety of organic compounds (for example, exhaust gases).
In the workplace, exposure to formaldehyde occurs in various ways. In its gaseous form, it is absorbed by the respiratory tract; in aqueous solution, it is absorbed through skin contact. The health effects associated with exposure to this substance vary with the exposure route and the concentration or dose absorbed.
In extreme situations such as accidents, formaldehyde may be present at high concentrations in the air, representing a considerable immediate danger. Concentrations equal to or greater than 20 ppm can cause serious pulmonary oedema and eventually death. In the case of direct skin contact, formaldehyde may produce skin lesions such as irritation, irritant contact dermatitis and allergic contact dermatitis. The symptoms are itching, tingling and redness. Skin sensitization is likely to appear after contact with aqueous solutions of formaldehyde at concentrations equal to or greater than 2%, or even solids or resins containing free formaldehyde. When someone is sensitized, skin allergy (erythema) symptoms may occur at every contact with solutions of increasingly lower concentration (starting at 0.5 percent formaldehyde). These effects are easily avoidable by protecting exposed skin for example, by wearing gloves.
Following exposure to contaminated air, the first effect is irritation of the mucous membranes of the eye and upper respiratory tract (nose and throat). The related symptoms are tingling, redness or burns to the nose and throat, nasal discharge and watery eyes. These symptoms are generally negligible to slight for formaldehyde concentrations below 1 ppm. They can become bothersome and even intolerable at higher concentrations mainly when they exceed 2 to 3 ppm.
In rare cases, formaldehyde causes sensitizing or allergic type changes in lung function. These are manifested by a decrease in lung capacity and by asthma attacks likely to recur at decreasing concentrations. These effects were observed with asthmatic and non-asthmatic subjects exposed to more than 2 ppm.(2) Nevertheless, there is no consensus in scientific literature that asthmatics have a more severe reaction to formaldehyde exposure than non-asthmatics. The allergenic effect of formaldehyde can be worsened by the presence of particles or dust (for example, wood dust), that trigger bronchial reactions even at concentrations below 2 ppm.
See also
Notes
- ↑ J. Read. Text-Book of Organic Chemistry, G Bell & Sons, (London, 1935).
- ↑ Drugs Forum, Marquis. Retrieved July 27, 2016.
- ↑ National Center for Biotechnology Information, Health effects of low-level exposure to formaldehyde. Retrieved July 27, 2016.
- ↑ EPA, Testing for Indoor Air Quality Section 01 81 09, December 2007. Retrieved July 27, 2016.
- ↑ IARC, IARC Classifies Formaldehyde as Carcinogenic to Humans. Press Release N° 153, June 15, 2004. Retrieved July 27, 2016.
- ↑ DermNetNZ, Formaldehyde allergy. Retrieved July 27, 2016.
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0136436692.
- Read. J. Text-Book of Organic Chemistry. London: G Bell & Sons, 1935.
- Solomons, T.W. Graham, and Craig B. Fryhle. Organic Chemistry 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998.
External links
All links retrieved April 1, 2024.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
