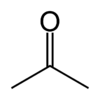
Acetone
| Acetone | |
|---|---|
| |
| General | |
| Systematic name | Propanone |
| Other names | β-ketopropane Dimethyl ketone, |
| Molecular formula | CH3COCH3 |
| SMILES | CC(=O)C |
| Molar mass | 58.09 g/mol |
| Appearance | Colorless liquid |
| CAS number | [67-64-1] |
| Properties | |
| Density and phase | 0.79 g/cm³, liquid |
| Solubility in water | miscible |
| Melting point | −94.9 °C (178.2 K) |
| Boiling point | 56.3 °C (329.4 K) |
| Viscosity | 0.32 cP at 20 °C |
| Structure | |
| Molecular shape | trigonal planar at C=O |
| Dipole moment | 2.91 D |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Flammable (F) Irritant (Xi) |
| NFPA 704 | |
| R-phrases | R11, R36, R66, R67 |
| S-phrases | S2, S9, S16, S26 |
| Flash point | −20 °C |
| Flammable limits in air (by volume) |
2.55% - 12.80% |
| Autoignition temperature | 465 °C |
| RTECS number | AL31500000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related ketones | Butanone |
| Related solvents | Water Ethanol Isopropanol Toluene |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Acetone (also known as propanone, dimethyl ketone, 2-propanone, propan-2-one and β-ketopropane) is the simplest representative of the group of chemical compounds known as ketones. It is a colorless, volatile, flammable liquid. In addition to being manufactured as a chemical, acetone is also found naturally in the environment, including in small amounts in the human body.
Acetone is a highly effective solvent for many organic compounds and is the active ingredient in nail polish remover. It is also used to make various plastics, fibers, drugs, and other chemicals.
Occurrence in the human body
Small amounts of acetone are metabolically produced in the body, mainly from fat. Fasting significantly increases its endogenous production (see ketosis). Acetone can be elevated in cases of diabetes.
Chemical synthesis
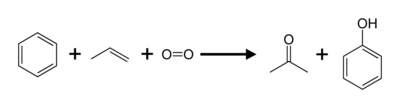
Most of the worldwide industrial production of acetone (and phenol) is currently based on a method called the cumene process. This process converts two relatively cheap starting materials, benzene and propylene, into acetone and phenol. Another reactant is oxygen (from the air).[1] The reaction is named after cumene (isopropyl benzene), the intermediate material formed during the process.
The overall reaction may be written as follows:
Before the invention of the cumene process, acetone was produced by the dry distillation of acetates, such as calcium acetate.
During World War I, a method of producing acetone through bacterial fermentation was developed by Chaim Weizmann, (who later became the first president of Israel), to help the British war effort.
Characteristics
Acetone has a melting point of −95.4 °C and boiling point of 56.53 °C. It has a relative density of 0.819 (at 0 °C). It acts as a solvent and is readily miscible with other solvents, including water, ethanol, and diethyl ether.
Uses
- An important industrial use for acetone involves its reaction with phenol to produce bisphenol A, which is a valuable component of many polymers, such as polycarbonates, polyurethanes, and epoxy resins.
- Another industrial application involves its use as a general purpose cleaner in paint and ink manufacturing operations.
- Acetone is also used extensively for the safe transport and storage of acetylene. Vessels containing a porous material are first filled with acetone followed by acetylene, which dissolves into the acetone. One liter of acetone can dissolve around 250 liters of acetylene.
- It is often the primary (or only) component in nail polish remover.[2] It is also used as a superglue remover. It can be used for thinning and cleaning fiberglass resins and epoxies, and it is highly effective in removing stains by permanent markers.
- It can be used as an agent in art work. When rubbed on the back of any laser print or laser photocopy, it produces a rough ready effect.
- It has been used in the manufacture of cordite.
- It is a strong solvent for most plastics (including those used in consumer-targeted water bottles) and synthetic fibers.
- It is used as a drying agent, as it is readily miscible with water and is volatile.
- In the laboratory, acetone is used as a polar aprotic solvent (solvent that does not release hydrogen ions) in a variety of organic reactions.
Health effects
At relatively low concentrations, acetone is not very toxic. It can, however, irritate and damage skin and the mucosal lining of the mouth. Its fumes should be avoided, as inhalation may lead to liver damage. In addition, one should always wear goggles when handling the substance, as it can cause permanent eye damage (corneal clouding).
Contamination of water, food (such as milk), or air (by acetone vapors) can lead to chronic exposure to acetone. A number of acute poisoning cases have been described. Accidental intake of large amounts of acetone may lead to unconsciousness and death.
Animal studies have shown that long-term exposure to acetone can damage the kidneys, liver, and nerves, increase birth defects, and lower the reproductive capacity of males (only). It is not known if the same effects would occur in humans. Pregnant women should avoid contact with acetone and its fumes, to avoid the possibility of birth defects, including brain damage.
Interestingly, acetone has been shown to have anticonvulsant effects in animal models of epilepsy, in the absence of toxicity, when administered at low (millimolar) concentrations.[3] It has been hypothesized that the high-fat, low-carbohydrate, ketogenic diet used clinically to control drug-resistant epilepsy in children works by elevating acetone in the brain.[3]
Chemical safety measures
Due to chemical incompatibilities, it is recommended that acetone should be kept away from bromine, chlorine, nitric acid, sulfuric acid and trichloromethane.
See also
Notes
- ↑ A small amount of a radical initiator is added to start the reaction.
- ↑ Acetonitrile, another organic solvent, is sometimes used as well.
- ↑ 3.0 3.1 Likhodii et al., Anticonvulsant properties of acetone, a brain ketone elevated by the ketogenic diet Ann Neurol., 54(2) (2003): 219–226. Retrieved April 3, 2018.
ReferencesISBN links support NWE through referral fees
- McMurry, John. Organic Chemistry, 6th ed. Belmont, CA: Brooks/Cole, 2004. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. Organic Chemistry, 6th ed. Englewood Cliffs, NJ: Prentice Hall, 1992. ISBN 0136436692.
- Solomons, T. W. Graham, and Craig B. Fryhle. Organic Chemistry, 8th ed. Hoboken, NJ: John Wiley, 2004. ISBN 0471417998.
External links
All links retrieved June 14, 2023.
- Aldehydes and Ketones Virtual Textbook of Organic Chemistry.
- Acetone. NIOSH Pocket Guide to Chemical Hazards.
- Acetone NIST Chemistry WebBook.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.