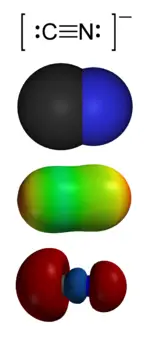Difference between revisions of "Cyanide" - New World Encyclopedia
Rick Swarts (talk | contribs) |
|||
| (11 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
| − | [[Image:Cyanide-montage.png|thumb|right|150px|The '''cyanide''' ion, CN<sup>−</sup>.<br> | + | {{Images OK}}{{Approved}}{{copyedited}} |
| − | From the top:<br> | + | [[Image:Cyanide-montage.png|thumb|right|150px|The '''cyanide''' ion, CN<sup>−</sup>.<br/> |
| − | 1. Valence-bond structure<br> | + | From the top:<br/> |
| − | 2. [[Space-filling model]]<br> | + | 1. Valence-bond structure<br/> |
| − | 3. Electrostatic potential surface<br> | + | 2. [[Space-filling model]]<br/> |
| + | 3. Electrostatic potential surface<br/> | ||
4. 'Carbon lone pair' [[HOMO/LUMO|HOMO]]]] | 4. 'Carbon lone pair' [[HOMO/LUMO|HOMO]]]] | ||
| − | '''Cyanide''' is any [[chemical compound]] containing a [[nitrile|cyano group]] (C≡N), which consists of a [[carbon]] [[atom]] [[chemical bond|triple-bonded]] to a [[nitrogen]] atom. Specifically, cyanide is the [[anion]] CN<sup>-</sup>. The various cyanides are salts or esters of HCN (hydrogen cyanide or hydrocyanic acid), whereby the hydrogen is replaced with a metal or radical, yielding such as potassium cyanide (KCN), calcium cyanide (CA(CN)<sub>2</sub>), or ethyl cyanide (CH<sub>3</sub>CH<sub>2</sub>CN). [[Organic compound]]s that feature cyanide as a functional group (responsible for the characteristic chemical reactions of those molecules) are called | + | '''Cyanide''' is any [[chemical compound]] containing a [[nitrile|cyano group]] (C≡N), which consists of a [[carbon]] [[atom]] [[chemical bond|triple-bonded]] to a [[nitrogen]] atom. Specifically, cyanide is the [[anion]] CN<sup>-</sup>. The various cyanides are salts or esters of HCN (hydrogen cyanide or hydrocyanic acid), whereby the hydrogen is replaced with a metal or radical, yielding such as potassium cyanide (KCN), calcium cyanide (CA(CN)<sub>2</sub>), or ethyl cyanide (CH<sub>3</sub>CH<sub>2</sub>CN). [[Organic compound]]s that feature cyanide as a functional group (responsible for the characteristic chemical reactions of those molecules) are called nitriles in [[IUPAC]] nomenclature. For example, CH<sub>3</sub>CN is referred to by the names [[acetonitrile]] or ethanenitrile per IUPAC, but occasionally it is labeled using the common name [[methyl]] cyanide. |
| − | Of the many kinds of cyanide compounds, some are | + | Of the many kinds of cyanide compounds, some are [[gas]]es, while others are solids or liquids. Those that can release the cyanide [[ion]] CN<sup>-</sup> are highly toxic. |
| − | The various cyanides have numerous commercial uses, including extracting gold and silver from ore, insecticides, exterminating pests such as | + | For [[plant]]s, cyanide offers an effective chemical defense against herbivores. Remarkably, it occurs naturally in a large number of popular food plants for people, such as [[cassava]], [[mango]]es, and [[almond]]s (as well as in apple seeds, wild cherry pits). However, human beings have the unique skill of pre-ingestion food processing that can overcome this toxicity, as well as a physiological ability to satisfactorily detoxify cyanide with a sufficient protein diet generally, allowing them to consume such foods (Jones 1998). For example, bitter almonds (as opposed to sweet almonds) can yield dangerous amounts of prussic acid (hydrogen cyanide) when eaten raw, but the toxicity can be removed by heating. |
| + | |||
| + | The various cyanides have numerous commercial uses, including extracting gold and silver from ore, use as insecticides, exterminating pests such as [[rat]]s, production of acrylic fibers and synthetic rubbers, and even for collecting [[fish]] for the aquarium trade. Most cyanides are toxic to humans and have been used as chemical weapons, including by Iraqi dictator Saddam Hussein against the Kurds and by [[Nazi Germany]] as an agent of [[genocide]] in death camps (Lerner and Lerner 2004). It also is a toxin found in cigarette smoke. | ||
==Overview== | ==Overview== | ||
| − | A cyanide ion is a negative ion with the formula CN<sup>−</sup>. The -CN group is sometimes referred to as a ''cyanide group'' or ''cyano group'' and compounds with them are sometimes referred to as cyanides. | + | A cyanide ion is a negative ion with the formula CN<sup>−</sup>. The -CN group is sometimes referred to as a ''cyanide group'' or ''cyano group'' and compounds with them are sometimes referred to as cyanides. In the -CN group, the [[carbon]] atom and the [[nitrogen]] atom are [[triple bond]]ed together. The prefix ''cyano'' is used in chemical nomenclature to indicate the presence of a nitrile group in a molecule. The -C'''≡'''N functional group is called a ''nitrile group''. |
Cyanide is considered, in a broad sense, to be the most potent [[ligand]] for many transition [[metal]]s. The very high affinities of metals for cyanide can be attributed to its negative charge, compactness, and ability to engage in π-bonding. This is responsible for many of the commercial uses of cyanides. Well known complexes include: | Cyanide is considered, in a broad sense, to be the most potent [[ligand]] for many transition [[metal]]s. The very high affinities of metals for cyanide can be attributed to its negative charge, compactness, and ability to engage in π-bonding. This is responsible for many of the commercial uses of cyanides. Well known complexes include: | ||
| − | * | + | *Hexacyanides [M(CN)<sub>6</sub>]<sup>3−</sup> (M = Ti, V, Cr, Mn, Fe, Co), which are octahedral in shape |
| − | * | + | *The tetracyanides, [M(CN)<sub>4</sub>]<sup>2−</sup> (M = Ni, Pd, Pt), which are square planar in their geometry |
| − | * | + | *The dicyanides [M(CN)<sub>2</sub>]<sup>−</sup> (M = Cu, Ag, Au), which are linear in geometry |
| − | The deep [[blue]] pigment [[Prussian blue]], used in the making of [[blueprint]]s, is derived from [[iron]] cyanide complexes. The word "cyanide" was extracted from "[[ferrocyanide]]" | + | The deep [[blue]] pigment [[Prussian blue]], used in the making of [[blueprint]]s, is derived from [[iron]] cyanide complexes. The word "cyanide" was extracted from "[[ferrocyanide]]," which proved to be a compound of iron and what is now known as the cyanide ion. Ferrocyanides and [[ferricyanide]]s were first discovered as Prussian blue, and were so named because Prussian blue contains iron and is blue; κυανεος is Greek for "blue" (Senning 2006). Prussian blue can produce hydrogen cyanide when exposed to acids. |
| − | As [[salt]]s or [[ester]]s of hydrogen cyanide (HCN, or hydrocyanic acid), cyanides are formed by replacing the hydrogen of hydrogen cyanide with a metal, such as sodium or potassium, or by replacing the hydrogen with a radical (such as ammonium). | + | As [[salt]]s or [[ester]]s of hydrogen cyanide (HCN, or hydrocyanic acid), cyanides are formed by replacing the hydrogen of hydrogen cyanide with a metal, such as [[sodium]] or [[potassium]], or by replacing the hydrogen with a radical (such as ammonium). |
| − | [[Hydrogen cyanide]] (HCN) is a colorless [[gas]] or highly volatile liquid that boils at | + | [[Hydrogen cyanide]] (HCN) is a colorless [[gas]] or highly volatile liquid that boils at 26°C (78.8°F), and is a weak acid. It has a faint, bitter, [[almond]]-like odor. Most people can smell hydrogen cyanide; however, due to an apparent [[genetics|genetic]] trait, some individuals cannot (OMIM 1994). |
Sodium and potassium cyanide are particularly common and widely used cyanides. [[Sodium cyanide]] and [[potassium cyanide]] are both white [[Powder (substance)|powders]] with a bitter [[almond]]-like odor in damp air, due to the presence of hydrogen cyanide formed by [[hydrolysis]]: | Sodium and potassium cyanide are particularly common and widely used cyanides. [[Sodium cyanide]] and [[potassium cyanide]] are both white [[Powder (substance)|powders]] with a bitter [[almond]]-like odor in damp air, due to the presence of hydrogen cyanide formed by [[hydrolysis]]: | ||
| Line 32: | Line 35: | ||
Cyanides are produced by certain [[bacterium|bacteria]], [[fungi]], and [[algae]] and are found in a number of foods and plants. Cyanide is found, although in small amounts, in [[apple]] seeds, [[mango]]es, and [[almonds]] (ATSDR 2006). | Cyanides are produced by certain [[bacterium|bacteria]], [[fungi]], and [[algae]] and are found in a number of foods and plants. Cyanide is found, although in small amounts, in [[apple]] seeds, [[mango]]es, and [[almonds]] (ATSDR 2006). | ||
| − | In plants, cyanides are usually bound to [[sugar]] molecules in the form of [[glycoside|cyanogenic glycosides]] and serve the plant as defense against [[herbivore]]s. [[Cassava]] roots (or manioc), an important [[potato]]-like food grown in tropical countries (and the base from which [[tapioca]] is made), contains cyanogenic glycosides (Vetter 2000; Jones 1998). Tapioca and cassava contain relatively low amounts of cyanide (ATSDR 2006), and foods such as cassava in combination with another chemical produces a vitamin B (Lerner and Lerner 2004). However, even in small quantities cyanide can be harmful, as see by the fact that diets heavy in cassava, such as parts of Africa, can | + | In plants, cyanides are usually bound to [[sugar]] molecules in the form of [[glycoside|cyanogenic glycosides]] and serve the plant as defense against [[herbivore]]s. [[Cassava]] roots (or manioc), an important [[potato]]-like food grown in tropical countries (and the base from which [[tapioca]] is made), contains cyanogenic glycosides (Vetter 2000; Jones 1998). [[Tapioca]] and cassava contain relatively low amounts of cyanide (ATSDR 2006), and foods such as cassava in combination with another chemical produces a vitamin B (Lerner and Lerner 2004). However, even in small quantities cyanide can be harmful, as see by the fact that diets heavy in cassava, such as parts of Africa, can cause deaths from poisoning (Lerner and Lerner 2004). |
| − | The Fe-only and [NiFe]-[[hydrogenase]] [[enzyme]]s contain cyanide [[ligand]]s at their active sites. | + | Unlike sweet almonds, which may be eaten raw, bitter almonds may yield in the presence of water from six to eight percent of hydrogen cyanide (prussic acid). Extract of bitter almond was once used medicinally, but even in small doses effects are severe and in larger doses can be deadly (Cantor et al. 2006). The prussic acid (hydrogen cyanide) must be removed before consumption. |
| + | |||
| + | The Fe-only and [NiFe]-[[hydrogenase]] [[enzyme]]s contain cyanide [[ligand]]s at their active sites. The biosynthesis of cyanide in the [NiFe]-hydrogenases proceeds from carbamoylphosphate, which converts to cysteinyl [[thiocyanate]], the CN<sup>-</sup> donor (Reissmann et al. 2003). | ||
Hydrogen cyanide is a product of certain kinds of [[pyrolysis]] and consequently it occurs in the exhaust of [[internal combustion engine]]s, [[tobacco]] smoke, and certain [[plastic]]s, especially those derived from [[acrylonitrile]]. | Hydrogen cyanide is a product of certain kinds of [[pyrolysis]] and consequently it occurs in the exhaust of [[internal combustion engine]]s, [[tobacco]] smoke, and certain [[plastic]]s, especially those derived from [[acrylonitrile]]. | ||
==Organic synthesis== | ==Organic synthesis== | ||
| − | Because of its high [[nucleophile|nucleophilicity]], cyanide is readily introduced into organic molecules by displacement of a [[halide]] group ( | + | Because of its high [[nucleophile|nucleophilicity]], cyanide is readily introduced into organic molecules by displacement of a [[halide]] group (that is, the [[chloride]] on [[methyl chloride]]). Organic cyanides are generally called nitriles. Thus, CH<sub>3</sub>CN can be called methyl cyanide but more commonly is referred to as [[acetonitrile]]. |
In organic synthesis, cyanide is used as a C-1 [[synthon]]. In other words, it can be used to lengthen a carbon chain by one, while retaining the ability to be functionalized. | In organic synthesis, cyanide is used as a C-1 [[synthon]]. In other words, it can be used to lengthen a carbon chain by one, while retaining the ability to be functionalized. | ||
| Line 51: | Line 56: | ||
==Toxicity== | ==Toxicity== | ||
| + | Many cyanide-containing compounds are highly toxic, but some are not. [[Prussian blue]], with an approximate formula Fe<sub>7</sub>(CN)<sub>18</sub>, is the blue of [[blue print]]s and is administered orally as an antidote to poisoning by [[thallium]] and [[Caesium-137]]. The most dangerous cyanides are [[hydrogen cyanide]] (HCN) and salts derived from it, such as potassium cyanide (KCN) and sodium cyanide (NaCN), among others. Also some compounds readily release HCN or the cyanide ion, such as [[trimethylsilyl cyanide]] (CH<sub>3</sub>)<sub>3</sub>SiCN upon contact with water and [[cyanoacrylate]]s upon [[pyrolysis]]. | ||
| − | + | Cyanides act as a toxin by causing respiratory failure, preventing the body's cells from receiving oxygen and particularly effecting the heart and brain which particularly depend on an oxygen supply (Lerner and Lerner 2004). Within minutes, cyanide poisoning can result in such symptoms as breathing rapidly, restlessness, dizziness, weakness, nausea, [[vomit]]ing, [[headache]], and rapid heart rate (Lerner and Lerner 2004). Larger amounts can cause rapid convulsions, lowering of heart rate and blood pressure, loss of consciousness, lung injury, and a respiratory failure that leads to death (Lerner and Lerner 2004). | |
| − | |||
| − | Cyanides act as a toxin by causing respiratory failure, preventing the body's cells from receiving oxygen and particularly effecting the heart and brain which particularly depend on an oxygen supply (Lerner and Lerner 2004). Within minutes, cyanide poisoning can result in such symptoms as breathing rapidly, restlessness, dizziness, weakness, nausea, | ||
| − | Specifically, cyanide is an [[enzyme inhibitor|inhibitor]] of the [[enzyme]] [[cytochrome c oxidase]] (also known as aa<sub>3</sub>) in the fourth complex of the [[electron transport chain]] (found in the membrane of the [[mitochondria]] of eukaryotic cells.) It attaches to the iron within this protein. | + | Specifically, cyanide is an [[enzyme inhibitor|inhibitor]] of the [[enzyme]] [[cytochrome c oxidase]] (also known as aa<sub>3</sub>) in the fourth complex of the [[electron transport chain]] (found in the membrane of the [[mitochondria]] of eukaryotic cells.) It attaches to the iron within this protein. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce [[Adenosine triphosphate|ATP]] for energy. Tissues that mainly depend on [[aerobic respiration]], such as the [[central nervous system]] and the [[heart]], are particularly affected. |
| − | Antidotes to cyanide poisoning include [[hydroxocobalamin]] and [[sodium nitrite]] which release the cyanide from the cytochrome system, and [[rhodanase]], which is an enzyme occurring naturally in mammals that combines serum cyanide with thiosulfate, producing comparatively harmless thiocyanate. | + | Antidotes to cyanide poisoning include [[hydroxocobalamin]] and [[sodium nitrite]] which release the cyanide from the cytochrome system, and [[rhodanase]], which is an enzyme occurring naturally in mammals that combines serum cyanide with thiosulfate, producing comparatively harmless thiocyanate. |
==Applications== | ==Applications== | ||
| Line 67: | Line 71: | ||
=== Fishing === | === Fishing === | ||
| − | + | Cyanides are illegally used to capture live fish near [[coral reef]]s for the [[aquarium]] and seafood markets. This fishing occurs mainly in the [[Philippines]], [[Indonesia]], and the [[Caribbean]] to supply the 2 million marine aquarium owners in the world. In this method, a diver uses a large, needleless [[syringe]] to squirt a cyanide solution into areas where the fish are hiding, stunning them so that they can be easily gathered. Many fish caught in this fashion die immediately, or in shipping. Those that survive to find their way into pet stores often die from shock, or from massive digestive damage. The high concentrations of cyanide on reefs on which this has occurred has resulted in cases of cyanide poisoning among local fishers and their families, as well as damage to the coral reefs themselves and other marine life in the area. | |
| − | Cyanides are illegally used to capture live fish near [[coral reef]]s for the [[aquarium]] and seafood markets. | ||
===Insecticide and pesticide=== | ===Insecticide and pesticide=== | ||
| Line 76: | Line 79: | ||
[[Gold]] and [[silver]] cyanides are among the very few [[soluble]] forms of these metals, and cyanides are thus used in [[mining]] as well as [[electroplating]], [[metallurgy]], [[jewelry]], and [[photography]]. | [[Gold]] and [[silver]] cyanides are among the very few [[soluble]] forms of these metals, and cyanides are thus used in [[mining]] as well as [[electroplating]], [[metallurgy]], [[jewelry]], and [[photography]]. | ||
| − | In the ''[[cyanide process]]'' | + | In the ''[[cyanide process]],'' finely ground high-grade gold or silver ore is mixed with cyanide (concentration of about two kilogram NaCN per metric ton); low-grade ores are stacked into heaps and sprayed with cyanide solution (concentration of about one kilogram NaCN per ton). The precious-metal [[cation]]s are complexed by the cyanide [[anion]]s to form soluble derivatives, such as [Au(CN)<sub>2</sub>]<sup>−</sup> and [Ag(CN)<sub>2</sub>]<sup>−</sup>. |
::2 Au + 4 KCN + ½ O<sub>2</sub> + H<sub>2</sub>O → 2 K[Au(CN)<sub>2</sub>] + 2 KOH | ::2 Au + 4 KCN + ½ O<sub>2</sub> + H<sub>2</sub>O → 2 K[Au(CN)<sub>2</sub>] + 2 KOH | ||
::2 Ag + 4 KCN + ½ O<sub>2</sub> + H<sub>2</sub>O → 2 K[Ag(CN)<sub>2</sub>] + 2 KOH | ::2 Ag + 4 KCN + ½ O<sub>2</sub> + H<sub>2</sub>O → 2 K[Ag(CN)<sub>2</sub>] + 2 KOH | ||
| Line 82: | Line 85: | ||
Silver is less "noble" than gold and often occurs as the sulfide, in which case redox is not invoked (no O<sub>2</sub> is required), instead a displacement reaction occurs: | Silver is less "noble" than gold and often occurs as the sulfide, in which case redox is not invoked (no O<sub>2</sub> is required), instead a displacement reaction occurs: | ||
::Ag<sub>2</sub>S + 4 KCN → 2 K[Ag(CN)<sub>2</sub>] + K<sub>2</sub>S | ::Ag<sub>2</sub>S + 4 KCN → 2 K[Ag(CN)<sub>2</sub>] + K<sub>2</sub>S | ||
| − | The "pregnant liquor" containing these ions is separated from the solids, which are discarded to a tailing pond or spent heap, the recoverable gold having been removed. | + | The "pregnant liquor" containing these ions is separated from the solids, which are discarded to a tailing pond or spent heap, the recoverable gold having been removed. The metal is recovered from the "pregnant solution" by reduction with [[zinc]] dust or by adsorption onto activated carbon. |
This process can result in environmental and health problems. Aqueous cyanide is hydrolyzed rapidly, especially in sunlight. It can mobilize some heavy metals such as mercury if present. Mercury has often been used in the refining process. The mercury can quickly more up the [[food chain]]. Gold can also be associated with arsenopyrite (FeAsS), which is similar to [[iron pyrite]] (fool's gold), wherein half of the sulfur atoms are replaced by [[arsenic]]. Au-containing arsenopyrite ores are similarly reactive toward cyanide. | This process can result in environmental and health problems. Aqueous cyanide is hydrolyzed rapidly, especially in sunlight. It can mobilize some heavy metals such as mercury if present. Mercury has often been used in the refining process. The mercury can quickly more up the [[food chain]]. Gold can also be associated with arsenopyrite (FeAsS), which is similar to [[iron pyrite]] (fool's gold), wherein half of the sulfur atoms are replaced by [[arsenic]]. Au-containing arsenopyrite ores are similarly reactive toward cyanide. | ||
===Color application to sculptures=== | ===Color application to sculptures=== | ||
| − | [[Potassium ferrocyanide]] is used to achieve a blue color on cast [[bronze sculpture]]s during the final finishing stage of the sculpture. On its own, it will produce a very dark shade of blue and is often mixed with other chemicals to achieve the desired tint and hue. It is applied using a torch and paint brush while wearing the standard safety equipment used for any patina application: | + | [[Potassium ferrocyanide]] is used to achieve a blue color on cast [[bronze sculpture]]s during the final finishing stage of the sculpture. On its own, it will produce a very dark shade of blue and is often mixed with other chemicals to achieve the desired tint and hue. It is applied using a torch and paint brush while wearing the standard safety equipment used for any patina application: [[Rubber gloves]], [[safety glasses]], and a [[respirator]]. The actual amount of cyanide in the mixture varies according to the recipes used by each foundry. |
===Cyanide as a poison of humans=== | ===Cyanide as a poison of humans=== | ||
| − | + | Cyanide has been used as a [[poison]] many times throughout history. Its most infamous application was the use of [[hydrogen cyanide]] by the [[Nazi]] regime in Germany for mass murder in some [[gas chamber]]s during [[the Holocaust]]. In the Iran-Iraq war of the 1980s, [[Iraq]]i dictator [[Saddam Hussein]] used hydrogen cyanide as one of the [[chemical weapon]]s used in killing [[Kurdish people|Kurds]]. | |
| − | Cyanide has been used for murder, as in the case of [[Grigori Rasputin]]. It has also been used for suicide. Some notable cases are [[Erwin Rommel]], [[Eva Braun]], [[Wallace Carothers]], [[Hermann Göring]], [[Heinrich Himmler]], [[Alan Turing]], [[Odilo Globocnik]], [[Adolf Hitler]] (in combination with a gunshot), residents of [[Jim Jones]]' the [[People's Temple]] in [[Jonestown]], and the [[Liberation Tigers of Tamil Eelam]] (they use it to kill themselves if they are captured by | + | Cyanide has been used for [[murder]], as in the case of [[Grigori Rasputin]]. It has also been used for [[suicide]]. Some notable cases are [[Erwin Rommel]], [[Eva Braun]], [[Wallace Carothers]], [[Hermann Göring]], [[Heinrich Himmler]], [[Alan Turing]], [[Odilo Globocnik]], [[Adolf Hitler]] (in combination with a gunshot), residents of [[Jim Jones]]' the [[People's Temple]] in [[Jonestown]], and the [[Liberation Tigers of Tamil Eelam]] (they use it to kill themselves if they are captured by armed forces). Individuals working in [[espionage]] would take cyanide in crystal form that could be taken if captured. |
==Chemical tests for cyanide== | ==Chemical tests for cyanide== | ||
====Prussian blue==== | ====Prussian blue==== | ||
| − | The formation of [[Prussian blue]] can be used as a test for inorganic [[cyanide]], for instance in the [[sodium fusion test]]. | + | The formation of [[Prussian blue]] can be used as a test for inorganic [[cyanide]], for instance in the [[sodium fusion test]]. Typically, [[iron(II) sulfate]] is added to a solution suspected of containing cyanide, such as the filtrate from the sodium fusion test. The resulting mixture is acidified with mineral acid. The formation of Prussian blue is a positive result for cyanide. |
| − | ===='' | + | ====''Para''-benzoquinone in DMSO==== |
A solution of ''para''-[[benzoquinone]] in [[dimethyl sulfoxide|DMSO]] reacts with cyanide to form a cyano[[phenol]], which is [[fluorescent]]. Illumination with a [[UV light]] gives a green/blue glow if the test is positive. | A solution of ''para''-[[benzoquinone]] in [[dimethyl sulfoxide|DMSO]] reacts with cyanide to form a cyano[[phenol]], which is [[fluorescent]]. Illumination with a [[UV light]] gives a green/blue glow if the test is positive. | ||
====Copper and an aromatic amine==== | ====Copper and an aromatic amine==== | ||
| − | As used by [[fumigation|fumigators]] to detect [[hydrogen cyanide]], [[copper]](II) salt and an aromatic amine such as [[benzidine]] is added to the sample; as an alternative to the benzidine, an alternative amine di-(4,4-''bis''-dimethylaminophenyl) methane can be used. A positive test gives a blue color. | + | As used by [[fumigation|fumigators]] to detect [[hydrogen cyanide]], [[copper]](II) salt and an aromatic amine such as [[benzidine]] is added to the sample; as an alternative to the benzidine, an alternative amine di-(4,4-''bis''-dimethylaminophenyl) methane can be used. A positive test gives a blue color. [[Copper(I) cyanide]] is poorly soluble. By [[sequestering]] the copper(I), the copper(II) is rendered a stronger [[oxidant]]. The copper, in a cyanide facilitated oxidation, converts the [[amine]] into a colored compound. The [[Nernst equation]] explains this process. Another good example of such chemistry is the way in which the saturated [[calomel]] [[reference electrode]] ([[saturated calomel electrode|SCE]]) works. The copper, in a cyanide facilitated, oxidation converts the amine into a colored compound. |
| − | ====Pyridine - Barbituric Acid Colorimetry ==== | + | ====Pyridine--Barbituric Acid Colorimetry ==== |
| − | A sample containing cyanide is purged with air from a boiling acid solution into a basic absorber solution. The cyanide salt absorbed in the basic solution is buffered at pH 4.5 and then reacted with [[chlorine]] to form cyanogen chloride. The cyanogen chloride formed couples pyridine with barbituric acid to form a strongly colored red dye that is proportional to cyanide concentration. This colorimetric method following distillation is the basis for most regulatory methods (for instance EPA 335.4) used to analyze cyanide in water, wastewater, and contaminated soils. Distillation followed by colorimetric methods, however, have been found to be prone to interferences from thiocyanate, nitrate, thiosulfate, sulfite, and sulfide that can result in both positive and negative bias. It has been recommended by the USEPA (MUR March 12, 2007) that samples containing these compounds be analyzed by Gas-Diffusion Flow Injection Analysis - Amperometry. | + | A sample containing cyanide is purged with air from a boiling acid solution into a basic absorber solution. The cyanide salt absorbed in the basic solution is buffered at pH 4.5 and then reacted with [[chlorine]] to form cyanogen chloride. The cyanogen chloride formed couples pyridine with barbituric acid to form a strongly colored red dye that is proportional to cyanide concentration. This colorimetric method following distillation is the basis for most regulatory methods (for instance EPA 335.4) used to analyze cyanide in water, wastewater, and contaminated soils. Distillation followed by colorimetric methods, however, have been found to be prone to interferences from thiocyanate, nitrate, thiosulfate, sulfite, and sulfide that can result in both positive and negative bias. It has been recommended by the USEPA (MUR March 12, 2007) that samples containing these compounds be analyzed by Gas-Diffusion Flow Injection Analysis--Amperometry. |
| − | ==== Gas | + | ==== Gas diffusion flow injection analysis--Amperometry ==== |
Instead of distilling, the sample is injected into an acidic stream where the HCN formed is passed under a hydrophobic gas diffusion membrane that selectively allows only HCN to pass through. The HCN that passes through the membrane is absorbed into a basic carrier solution that transports the CN to an amperometric detector that accurately measures cyanide concentration with high sensitivity. Sample pretreatment determined by acid reagents, ligands, or preliminary UV irradiation allow cyanide speciation of free cyanide, available cyanide, and total cyanide, respectively. The relative simplicity of these flow injection analysis methods limits the interference experienced by the high heat of distillation and also proves to be cost effective, since time consuming distillations are not required. | Instead of distilling, the sample is injected into an acidic stream where the HCN formed is passed under a hydrophobic gas diffusion membrane that selectively allows only HCN to pass through. The HCN that passes through the membrane is absorbed into a basic carrier solution that transports the CN to an amperometric detector that accurately measures cyanide concentration with high sensitivity. Sample pretreatment determined by acid reagents, ligands, or preliminary UV irradiation allow cyanide speciation of free cyanide, available cyanide, and total cyanide, respectively. The relative simplicity of these flow injection analysis methods limits the interference experienced by the high heat of distillation and also proves to be cost effective, since time consuming distillations are not required. | ||
==References== | ==References== | ||
| − | + | * Agency for Toxic Substances and Disease Registry (ATSDR). 2006. [http://www.atsdr.cdc.gov/tfacts8.html ToxFAQs™ for cyanide.] ''Agency for Toxic Substances and Disease Registry, Division of Toxicology and Environmental Medicine, [[CDC]]''. Retrieved August 3, 2008. | |
| − | Agency for Toxic Substances and Disease Registry (ATSDR). 2006. | + | * Cantor, D., J. Fleischer, J. Green, and D.L. Israel. 2006. "The fruit of the matter." ''Mental Floss'' 5(4): 12. |
| − | + | * Jones, D. A. 1998. [http://www.ncbi.nlm.nih.gov/pubmed/9431670 Why are so many food plants cyanogenic?] ''Phytochemistry'' 47: 155–162. Retrieved August 3, 2008. | |
| − | + | * Lerner, K.L., and B.W. Lerner. 2004. ''Encyclopedia of Espionage, Intelligence, and Security''. Detroit, MI: Thomson/Gale. ISBN 0787675466. | |
| − | + | * Online Mendelian Inheritance in Man (OMIM). 1994. [http://www.ncbi.nlm.nih.gov/entrez/dispomim.cgi?id=304300 Cyanide, inability to smell.] ''Johns Hopkins University''. Retrieved August 3, 2008. | |
| − | + | * Reissmann, S., E. Hochleitner, H. Wang, A. Paschos, F. Lottspeich, R.S. Glass, and A. Böck. 2003. [http://www.sciencemag.org/cgi/content/abstract/299/5609/1067 Taming of a poison: Biosynthesis of the NiFe-hydrogenase cyanide ligands.] ''Science'' 299(5609): 1067–1070. Retrieved August 3, 2008. | |
| − | + | * Senning, A. 2006. ''Elsevier's Dictionary of Chemoetymology''. Elsevier. ISBN 0444522395. | |
| − | * Lerner, K. L., and B. W. Lerner. 2004. ''Encyclopedia of Espionage, Intelligence, and Security''. Detroit, MI: Thomson/Gale. ISBN 0787675466 | + | * Takano, R. 1916. [http://www.jem.org/cgi/content/abstract/24/2/207 The treatment of leprosy with cyanocuprol]. ''The Journal of Experimental Medicine'' 24: 207–211. Retrieved August 3, 2008. |
| − | + | * Vetter, J. 2000. [http://www.ncbi.nlm.nih.gov/pubmed/10669009 Plant cyanogenic glycosides]. ''Toxicon'' 38: 11–36. Retrieved August 3, 2008. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | * | ||
| − | |||
| − | |||
Latest revision as of 21:50, 1 August 2011
Cyanide is any chemical compound containing a cyano group (C≡N), which consists of a carbon atom triple-bonded to a nitrogen atom. Specifically, cyanide is the anion CN-. The various cyanides are salts or esters of HCN (hydrogen cyanide or hydrocyanic acid), whereby the hydrogen is replaced with a metal or radical, yielding such as potassium cyanide (KCN), calcium cyanide (CA(CN)2), or ethyl cyanide (CH3CH2CN). Organic compounds that feature cyanide as a functional group (responsible for the characteristic chemical reactions of those molecules) are called nitriles in IUPAC nomenclature. For example, CH3CN is referred to by the names acetonitrile or ethanenitrile per IUPAC, but occasionally it is labeled using the common name methyl cyanide.
Of the many kinds of cyanide compounds, some are gases, while others are solids or liquids. Those that can release the cyanide ion CN- are highly toxic.
For plants, cyanide offers an effective chemical defense against herbivores. Remarkably, it occurs naturally in a large number of popular food plants for people, such as cassava, mangoes, and almonds (as well as in apple seeds, wild cherry pits). However, human beings have the unique skill of pre-ingestion food processing that can overcome this toxicity, as well as a physiological ability to satisfactorily detoxify cyanide with a sufficient protein diet generally, allowing them to consume such foods (Jones 1998). For example, bitter almonds (as opposed to sweet almonds) can yield dangerous amounts of prussic acid (hydrogen cyanide) when eaten raw, but the toxicity can be removed by heating.
The various cyanides have numerous commercial uses, including extracting gold and silver from ore, use as insecticides, exterminating pests such as rats, production of acrylic fibers and synthetic rubbers, and even for collecting fish for the aquarium trade. Most cyanides are toxic to humans and have been used as chemical weapons, including by Iraqi dictator Saddam Hussein against the Kurds and by Nazi Germany as an agent of genocide in death camps (Lerner and Lerner 2004). It also is a toxin found in cigarette smoke.
Overview
A cyanide ion is a negative ion with the formula CN−. The -CN group is sometimes referred to as a cyanide group or cyano group and compounds with them are sometimes referred to as cyanides. In the -CN group, the carbon atom and the nitrogen atom are triple bonded together. The prefix cyano is used in chemical nomenclature to indicate the presence of a nitrile group in a molecule. The -C≡N functional group is called a nitrile group.
Cyanide is considered, in a broad sense, to be the most potent ligand for many transition metals. The very high affinities of metals for cyanide can be attributed to its negative charge, compactness, and ability to engage in π-bonding. This is responsible for many of the commercial uses of cyanides. Well known complexes include:
- Hexacyanides [M(CN)6]3− (M = Ti, V, Cr, Mn, Fe, Co), which are octahedral in shape
- The tetracyanides, [M(CN)4]2− (M = Ni, Pd, Pt), which are square planar in their geometry
- The dicyanides [M(CN)2]− (M = Cu, Ag, Au), which are linear in geometry
The deep blue pigment Prussian blue, used in the making of blueprints, is derived from iron cyanide complexes. The word "cyanide" was extracted from "ferrocyanide," which proved to be a compound of iron and what is now known as the cyanide ion. Ferrocyanides and ferricyanides were first discovered as Prussian blue, and were so named because Prussian blue contains iron and is blue; κυανεος is Greek for "blue" (Senning 2006). Prussian blue can produce hydrogen cyanide when exposed to acids.
As salts or esters of hydrogen cyanide (HCN, or hydrocyanic acid), cyanides are formed by replacing the hydrogen of hydrogen cyanide with a metal, such as sodium or potassium, or by replacing the hydrogen with a radical (such as ammonium).
Hydrogen cyanide (HCN) is a colorless gas or highly volatile liquid that boils at 26°C (78.8°F), and is a weak acid. It has a faint, bitter, almond-like odor. Most people can smell hydrogen cyanide; however, due to an apparent genetic trait, some individuals cannot (OMIM 1994).
Sodium and potassium cyanide are particularly common and widely used cyanides. Sodium cyanide and potassium cyanide are both white powders with a bitter almond-like odor in damp air, due to the presence of hydrogen cyanide formed by hydrolysis:
- NaCN + H2O → HCN + NaOH
- KCN + H2O → HCN + KOH
Occurrence
Cyanides are produced by certain bacteria, fungi, and algae and are found in a number of foods and plants. Cyanide is found, although in small amounts, in apple seeds, mangoes, and almonds (ATSDR 2006).
In plants, cyanides are usually bound to sugar molecules in the form of cyanogenic glycosides and serve the plant as defense against herbivores. Cassava roots (or manioc), an important potato-like food grown in tropical countries (and the base from which tapioca is made), contains cyanogenic glycosides (Vetter 2000; Jones 1998). Tapioca and cassava contain relatively low amounts of cyanide (ATSDR 2006), and foods such as cassava in combination with another chemical produces a vitamin B (Lerner and Lerner 2004). However, even in small quantities cyanide can be harmful, as see by the fact that diets heavy in cassava, such as parts of Africa, can cause deaths from poisoning (Lerner and Lerner 2004).
Unlike sweet almonds, which may be eaten raw, bitter almonds may yield in the presence of water from six to eight percent of hydrogen cyanide (prussic acid). Extract of bitter almond was once used medicinally, but even in small doses effects are severe and in larger doses can be deadly (Cantor et al. 2006). The prussic acid (hydrogen cyanide) must be removed before consumption.
The Fe-only and [NiFe]-hydrogenase enzymes contain cyanide ligands at their active sites. The biosynthesis of cyanide in the [NiFe]-hydrogenases proceeds from carbamoylphosphate, which converts to cysteinyl thiocyanate, the CN- donor (Reissmann et al. 2003).
Hydrogen cyanide is a product of certain kinds of pyrolysis and consequently it occurs in the exhaust of internal combustion engines, tobacco smoke, and certain plastics, especially those derived from acrylonitrile.
Organic synthesis
Because of its high nucleophilicity, cyanide is readily introduced into organic molecules by displacement of a halide group (that is, the chloride on methyl chloride). Organic cyanides are generally called nitriles. Thus, CH3CN can be called methyl cyanide but more commonly is referred to as acetonitrile.
In organic synthesis, cyanide is used as a C-1 synthon. In other words, it can be used to lengthen a carbon chain by one, while retaining the ability to be functionalized.
- RX + CN− → RCN + X− (Nucleophilic Substitution) followed by
- RCN + 2 H2O → RCOOH + NH3 (Hydrolysis under reflux with mineral acid catalyst), or
- RCN + 0.5 LiAlH4 + (second step) 2 H2O → RCH2NH2 + 0.5 LiAl(OH)4 (under reflux in dry ether, followed by addition of H2O)
An alternative method for introducing cyanide is via the process of hydrocyanation, whereby hydrogen cyanide and alkenes combine: RCH=CH2 + HCN → RCH(CN)CH3 Metal catalysts are required for such reactions.
Toxicity
Many cyanide-containing compounds are highly toxic, but some are not. Prussian blue, with an approximate formula Fe7(CN)18, is the blue of blue prints and is administered orally as an antidote to poisoning by thallium and Caesium-137. The most dangerous cyanides are hydrogen cyanide (HCN) and salts derived from it, such as potassium cyanide (KCN) and sodium cyanide (NaCN), among others. Also some compounds readily release HCN or the cyanide ion, such as trimethylsilyl cyanide (CH3)3SiCN upon contact with water and cyanoacrylates upon pyrolysis.
Cyanides act as a toxin by causing respiratory failure, preventing the body's cells from receiving oxygen and particularly effecting the heart and brain which particularly depend on an oxygen supply (Lerner and Lerner 2004). Within minutes, cyanide poisoning can result in such symptoms as breathing rapidly, restlessness, dizziness, weakness, nausea, vomiting, headache, and rapid heart rate (Lerner and Lerner 2004). Larger amounts can cause rapid convulsions, lowering of heart rate and blood pressure, loss of consciousness, lung injury, and a respiratory failure that leads to death (Lerner and Lerner 2004).
Specifically, cyanide is an inhibitor of the enzyme cytochrome c oxidase (also known as aa3) in the fourth complex of the electron transport chain (found in the membrane of the mitochondria of eukaryotic cells.) It attaches to the iron within this protein. The binding of cyanide to this cytochrome prevents transport of electrons from cytochrome c oxidase to oxygen. As a result, the electron transport chain is disrupted, meaning that the cell can no longer aerobically produce ATP for energy. Tissues that mainly depend on aerobic respiration, such as the central nervous system and the heart, are particularly affected.
Antidotes to cyanide poisoning include hydroxocobalamin and sodium nitrite which release the cyanide from the cytochrome system, and rhodanase, which is an enzyme occurring naturally in mammals that combines serum cyanide with thiosulfate, producing comparatively harmless thiocyanate.
Applications
Cyanides have numerous commercial uses and some medicinal uses as well. Furthermore, it has been used as a chemical weapon and as a suicide pill in cases of espionage (Lerner and Lerner 2004). They have been used for production of plastics, synthetic rubbers, and acrylic fibers.
Medical uses
The cyanide compound sodium nitroprusside is occasionally used in emergency medical situations to produce a rapid decrease in blood pressure in humans; it is also used as a vasodilator in vascular research. The cobalt in artificial Vitamin B12 contains a cyanide ligand as an artifact of the purification process. During World War I, a copper cyanide compound was briefly used by Japanese physicians for the treatment of tuberculosis and leprosy (Takano 1916).
Fishing
Cyanides are illegally used to capture live fish near coral reefs for the aquarium and seafood markets. This fishing occurs mainly in the Philippines, Indonesia, and the Caribbean to supply the 2 million marine aquarium owners in the world. In this method, a diver uses a large, needleless syringe to squirt a cyanide solution into areas where the fish are hiding, stunning them so that they can be easily gathered. Many fish caught in this fashion die immediately, or in shipping. Those that survive to find their way into pet stores often die from shock, or from massive digestive damage. The high concentrations of cyanide on reefs on which this has occurred has resulted in cases of cyanide poisoning among local fishers and their families, as well as damage to the coral reefs themselves and other marine life in the area.
Insecticide and pesticide
Cyanides are used in pest control, as a fumigant in the storing of grain, and as an insecticide for the fumigating of ships. Cyanide salts have been used as rat poison, and for killing ants.
Mining and other commercial uses tied to gold and silver cyanides
Gold and silver cyanides are among the very few soluble forms of these metals, and cyanides are thus used in mining as well as electroplating, metallurgy, jewelry, and photography.
In the cyanide process, finely ground high-grade gold or silver ore is mixed with cyanide (concentration of about two kilogram NaCN per metric ton); low-grade ores are stacked into heaps and sprayed with cyanide solution (concentration of about one kilogram NaCN per ton). The precious-metal cations are complexed by the cyanide anions to form soluble derivatives, such as [Au(CN)2]− and [Ag(CN)2]−.
- 2 Au + 4 KCN + ½ O2 + H2O → 2 K[Au(CN)2] + 2 KOH
- 2 Ag + 4 KCN + ½ O2 + H2O → 2 K[Ag(CN)2] + 2 KOH
Silver is less "noble" than gold and often occurs as the sulfide, in which case redox is not invoked (no O2 is required), instead a displacement reaction occurs:
- Ag2S + 4 KCN → 2 K[Ag(CN)2] + K2S
The "pregnant liquor" containing these ions is separated from the solids, which are discarded to a tailing pond or spent heap, the recoverable gold having been removed. The metal is recovered from the "pregnant solution" by reduction with zinc dust or by adsorption onto activated carbon.
This process can result in environmental and health problems. Aqueous cyanide is hydrolyzed rapidly, especially in sunlight. It can mobilize some heavy metals such as mercury if present. Mercury has often been used in the refining process. The mercury can quickly more up the food chain. Gold can also be associated with arsenopyrite (FeAsS), which is similar to iron pyrite (fool's gold), wherein half of the sulfur atoms are replaced by arsenic. Au-containing arsenopyrite ores are similarly reactive toward cyanide.
Color application to sculptures
Potassium ferrocyanide is used to achieve a blue color on cast bronze sculptures during the final finishing stage of the sculpture. On its own, it will produce a very dark shade of blue and is often mixed with other chemicals to achieve the desired tint and hue. It is applied using a torch and paint brush while wearing the standard safety equipment used for any patina application: Rubber gloves, safety glasses, and a respirator. The actual amount of cyanide in the mixture varies according to the recipes used by each foundry.
Cyanide as a poison of humans
Cyanide has been used as a poison many times throughout history. Its most infamous application was the use of hydrogen cyanide by the Nazi regime in Germany for mass murder in some gas chambers during the Holocaust. In the Iran-Iraq war of the 1980s, Iraqi dictator Saddam Hussein used hydrogen cyanide as one of the chemical weapons used in killing Kurds.
Cyanide has been used for murder, as in the case of Grigori Rasputin. It has also been used for suicide. Some notable cases are Erwin Rommel, Eva Braun, Wallace Carothers, Hermann Göring, Heinrich Himmler, Alan Turing, Odilo Globocnik, Adolf Hitler (in combination with a gunshot), residents of Jim Jones' the People's Temple in Jonestown, and the Liberation Tigers of Tamil Eelam (they use it to kill themselves if they are captured by armed forces). Individuals working in espionage would take cyanide in crystal form that could be taken if captured.
Chemical tests for cyanide
Prussian blue
The formation of Prussian blue can be used as a test for inorganic cyanide, for instance in the sodium fusion test. Typically, iron(II) sulfate is added to a solution suspected of containing cyanide, such as the filtrate from the sodium fusion test. The resulting mixture is acidified with mineral acid. The formation of Prussian blue is a positive result for cyanide.
Para-benzoquinone in DMSO
A solution of para-benzoquinone in DMSO reacts with cyanide to form a cyanophenol, which is fluorescent. Illumination with a UV light gives a green/blue glow if the test is positive.
Copper and an aromatic amine
As used by fumigators to detect hydrogen cyanide, copper(II) salt and an aromatic amine such as benzidine is added to the sample; as an alternative to the benzidine, an alternative amine di-(4,4-bis-dimethylaminophenyl) methane can be used. A positive test gives a blue color. Copper(I) cyanide is poorly soluble. By sequestering the copper(I), the copper(II) is rendered a stronger oxidant. The copper, in a cyanide facilitated oxidation, converts the amine into a colored compound. The Nernst equation explains this process. Another good example of such chemistry is the way in which the saturated calomel reference electrode (SCE) works. The copper, in a cyanide facilitated, oxidation converts the amine into a colored compound.
Pyridine—Barbituric Acid Colorimetry
A sample containing cyanide is purged with air from a boiling acid solution into a basic absorber solution. The cyanide salt absorbed in the basic solution is buffered at pH 4.5 and then reacted with chlorine to form cyanogen chloride. The cyanogen chloride formed couples pyridine with barbituric acid to form a strongly colored red dye that is proportional to cyanide concentration. This colorimetric method following distillation is the basis for most regulatory methods (for instance EPA 335.4) used to analyze cyanide in water, wastewater, and contaminated soils. Distillation followed by colorimetric methods, however, have been found to be prone to interferences from thiocyanate, nitrate, thiosulfate, sulfite, and sulfide that can result in both positive and negative bias. It has been recommended by the USEPA (MUR March 12, 2007) that samples containing these compounds be analyzed by Gas-Diffusion Flow Injection Analysis—Amperometry.
Gas diffusion flow injection analysis—Amperometry
Instead of distilling, the sample is injected into an acidic stream where the HCN formed is passed under a hydrophobic gas diffusion membrane that selectively allows only HCN to pass through. The HCN that passes through the membrane is absorbed into a basic carrier solution that transports the CN to an amperometric detector that accurately measures cyanide concentration with high sensitivity. Sample pretreatment determined by acid reagents, ligands, or preliminary UV irradiation allow cyanide speciation of free cyanide, available cyanide, and total cyanide, respectively. The relative simplicity of these flow injection analysis methods limits the interference experienced by the high heat of distillation and also proves to be cost effective, since time consuming distillations are not required.
ReferencesISBN links support NWE through referral fees
- Agency for Toxic Substances and Disease Registry (ATSDR). 2006. ToxFAQs™ for cyanide. Agency for Toxic Substances and Disease Registry, Division of Toxicology and Environmental Medicine, CDC. Retrieved August 3, 2008.
- Cantor, D., J. Fleischer, J. Green, and D.L. Israel. 2006. "The fruit of the matter." Mental Floss 5(4): 12.
- Jones, D. A. 1998. Why are so many food plants cyanogenic? Phytochemistry 47: 155–162. Retrieved August 3, 2008.
- Lerner, K.L., and B.W. Lerner. 2004. Encyclopedia of Espionage, Intelligence, and Security. Detroit, MI: Thomson/Gale. ISBN 0787675466.
- Online Mendelian Inheritance in Man (OMIM). 1994. Cyanide, inability to smell. Johns Hopkins University. Retrieved August 3, 2008.
- Reissmann, S., E. Hochleitner, H. Wang, A. Paschos, F. Lottspeich, R.S. Glass, and A. Böck. 2003. Taming of a poison: Biosynthesis of the NiFe-hydrogenase cyanide ligands. Science 299(5609): 1067–1070. Retrieved August 3, 2008.
- Senning, A. 2006. Elsevier's Dictionary of Chemoetymology. Elsevier. ISBN 0444522395.
- Takano, R. 1916. The treatment of leprosy with cyanocuprol. The Journal of Experimental Medicine 24: 207–211. Retrieved August 3, 2008.
- Vetter, J. 2000. Plant cyanogenic glycosides. Toxicon 38: 11–36. Retrieved August 3, 2008.
| |||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.