Nitrile
A nitrile is any organic compound that has a -Câ¡N functional group. The -Câ¡N functional group is called a nitrile group. In the -CN group, the carbon and nitrogen atoms are linked to each other by what is called a "triple" covalent bond. To indicate the presence of a nitrile group in a molecule, chemists use the prefix cyano when naming the molecule.
The nitrile functional group needs to be distinguished from the cyanide ion. The latter is a negative ion with the formula CNâ. Yet the nitrile group is sometimes referred to as a cyanide group or cyano group, and compounds containing this group are sometimes referred to as cyanides. Under some conditions, nitriles may release the highly toxic cyanide (CNâ) ion.
Various nitriles serve important functions. For instance, benzonitrile is useful as a solvent and is a precursor for the synthesis of other materials. Another common nitrile, named acrylonitrile, can be converted to the polymer known as polyacrylonitrile, which makes up acrylic fibers. Acrylonitrile is also used in the synthesis of certain nylons and for the industrial manufacture of acrylamide and acrylic acid.
History
Hydrogen cyanide was first synthesized in 1782 by Carl Wilhelm Scheele, who was killed in an attempt to get the anhydrous compound.[1] Joseph Louis Gay-Lussac was the first to prepare the pure acid in 1811, and Friedrich Wöhler and Justus von Liebig were the first to prepare the nitriles benzoyl cyanide and benzonitrile in 1832. Théophile-Jules Pelouze synthesized propionitrile in 1834.
Synthesis of nitriles
Nitriles can be prepared by any of the following methods of organic chemistry:
- Reaction (nucleophilic aliphatic substitution) of an alkyl halide with a metal cyanide.
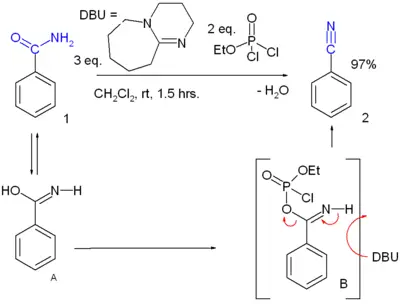
- Dehydration reaction of a primary amide. Many reagents are available. For instance, benzamide can be converted to benzonitrile[2]:
- Dehydration of secondary amides (von Braun amide degradation).
- Dehydration of aldoximes. (Possible reagents are triethylamine/sulfur dioxide, zeolites, or sulfuryl chloride.)
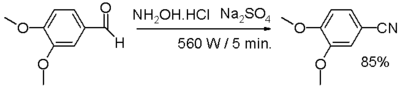
- One-pot synthesis from an aldehyde, with hydroxylamine and sodium sulfate.
- In one study,[3] an aromatic or aliphatic aldehyde is reacted with hydroxylamine and anhydrous sodium sulfate in a dry media reaction for a very small amount of time under microwave irradiation through an intermediate aldoxime.
- Reaction of a metal cyanide with an aldehyde in the cyanohydrin reaction.
- Derivation from aryl carboxylic acids (Letts nitrile synthesis).
- Aromatic nitriles from diazonium compounds in the Sandmeyer reaction.
Reactions of nitriles
Nitrile groups in organic compounds can undergo various reactions when subject to certain reactants or conditions. A nitrile group can be hydrolyzed, reduced, or ejected from a molecule as a cyanide ion.
- In hydrolysis, the nitrile is reacted with an acid and water at a high temperature, or with a base and water. The acid hydrolysis forms a carboxylic acid, the alkali hydrolysis forms a carboxylate.
- In organic reduction the nitrile is reduced by reacting it with hydrogen with a nickel catalyst; an amine is formed in this reaction. Reduction to the imine followed by hydrolysis to the aldehyde takes place in the Stephen aldehyde synthesis
- A nitrile is an electrophile at the carbon atom in a nucleophilic addition reactions:
- In "reductive decyanation," the nitrile group is replaced by a proton.[5] An effective decyanation is by a dissolving metal reduction with HMPA and potassium metal in tert-butyl alcohol. α-Amino-nitriles can be decyanated with lithium aluminum hydride.
- Nitriles self-react in the presence of a base in the Thorpe reaction (a nucleophilic addition).
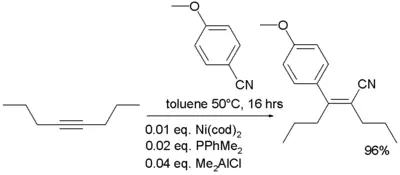
- In organometallic chemistry, nitriles are known to add to alkynes in a reaction known as carbocyanation[6]:
Uses of some nitriles
- Benzonitrile: Benzonitrile is a useful solvent and a versatile precursor to many derivatives.
- Acrylonitrile: Acrylonitrile is a valuable monomer for the manufacture of the polymer known as polyacrylonitrile, which makes up acrylic fibers. Dimerization of acrylonitrile produces adiponitrile, used in the synthesis of certain nylons. Small amounts of acrylonitrile are used as a fumigant. Acrylonitrile is also a precursor in the industrial manufacture of acrylamide and acrylic acid.
See also
Notes
- â David T. Mowry, "The Preparation of Nitriles," Chem. Rev. 42(2) (1948): 189â283; DOI:10.1021/cr60132a001. Retrieved August 19, 2007.
- â Chun-Wei Kuo, Jia-Liang Zhu, Jen-Dar Wu, Cheng-Ming Chu, Ching-Fa Yao and Kak-Shan Shia, "A Convenient New Procedure for Converting Primary Amides into Nitriles," Chem. Commun. (2007): 301â303; DOI:10.1039/b614061k. Retrieved August 19, 2007.
- â Sharwan K. Dewan, Ravinder Singh, and Anil Kumar, "One Pot Synthesis of Nitriles from Aldehydes and Hydroxylamine Hydrochloride Using Sodium Sulphate (anhyd) and Sodium Bicarbonate in Dry Media under Microwave Irradiation," Arkivoc (2006), (ii) 41-44; Online article. Retrieved August 19, 2007.
- â Andri L. Smith and Paula Tan, "Creatine Synthesis: An Undergraduate Organic Chemistry Laboratory Experiment," J. Chem. Educ. 83 (2006): 1654; Abstract. Retrieved August 19, 2007.
- â Jean-Marc Mattalia, Caroline Marchi-Delapierre, Hassan Hazimeh, and Michel Chanon, "The Reductive Decyanation Reaction: Chemical Methods and Synthetic Applications," Arkivoc (AL-1755FR) (2006): 90-118; Article. Retrieved August 19, 2007.
- â Yoshiaki Nakao, Akira Yada, Shiro Ebata, and Tamejiro Hiyama, "A Dramatic Effect of Lewis-Acid Catalysts on Nickel-Catalyzed Carbocyanation of Alkynes," J. Am. Chem. Soc. 129(9) (2007): 2428â2429. (Communication) DOI:10.1021/ja067364x. Retrieved August 19, 2007.
ReferencesISBN links support NWE through referral fees
- Daley, Richard F., and Daley, Sally J. 2005. Organic Chemistry. OChem4Free.com. Retrieved April 12, 2007.
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Boyd, Robert N. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0136436692.
- Solomons, T. W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
| Functional groups |
|---|
| Chemical class: Alcohol ⢠Aldehyde ⢠Alkane ⢠Alkene ⢠Alkyne ⢠Amide ⢠Amine ⢠Azo compound ⢠Benzene derivative ⢠Carboxylic acid ⢠Cyanate ⢠Ester ⢠Ether ⢠Haloalkane ⢠Imine ⢠Isocyanide ⢠Isocyanate ⢠Ketone ⢠Nitrile ⢠Nitro compound ⢠Nitroso compound ⢠Peroxide ⢠Phosphoric acid ⢠Pyridine derivative ⢠Sulfone ⢠Sulfonic acid ⢠Sulfoxide ⢠Thioether ⢠Thiol ⢠Toluene derivative |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.