Difference between revisions of "Uranium" - New World Encyclopedia
| (18 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{Copyedited}} |
{{Elementbox_header | number=92 | symbol=U | name=uranium | left=[[protactinium]] | right=[[neptunium]] | above=[[neodymium|Nd]] | below=(Uqb) | color1=#ff99cc | color2=black }} | {{Elementbox_header | number=92 | symbol=U | name=uranium | left=[[protactinium]] | right=[[neptunium]] | above=[[neodymium|Nd]] | below=(Uqb) | color1=#ff99cc | color2=black }} | ||
{{Elementbox_series | [[actinide]]s }} | {{Elementbox_series | [[actinide]]s }} | ||
| Line 19: | Line 19: | ||
{{Elementbox_section_atomicprop | color1=#ff99cc | color2=black }} | {{Elementbox_section_atomicprop | color1=#ff99cc | color2=black }} | ||
{{Elementbox_crystalstruct | orthorhombic }} | {{Elementbox_crystalstruct | orthorhombic }} | ||
| − | {{Elementbox_oxistates | 3+,4+,5+,6+<ref> | + | {{Elementbox_oxistates | 3+,4+,5+,6+<ref>L.R. Morss, N.M. Edelstein, J. Fuger, (eds.) ''The Chemistry of the Actinide and Transactinide Elements, Third Edition.'' (Netherlands: Springer, 2006.)</ref><br />(weakly [[base (chemistry)|basic]] oxide) }} |
{{Elementbox_electroneg_pauling | 1.38 }} | {{Elementbox_electroneg_pauling | 1.38 }} | ||
{{Elementbox_ionizationenergies2 | 597.6 | 1420 }} | {{Elementbox_ionizationenergies2 | 597.6 | 1420 }} | ||
| Line 59: | Line 59: | ||
'''Uranium''' (chemical symbol '''U''', [[atomic number]] 92) is a silvery [[metal]]lic [[chemical element]] in the [[actinide series]] of the [[periodic table]]. The heaviest naturally occurring element, uranium is nearly twice as dense as [[lead]] and weakly [[radioactivity|radioactive]]. It occurs naturally in low concentrations (a few parts per million) in soil, rock and water, and is commercially extracted from uranium-bearing [[mineral]]s such as [[uraninite]] (see [[uranium mining]]). | '''Uranium''' (chemical symbol '''U''', [[atomic number]] 92) is a silvery [[metal]]lic [[chemical element]] in the [[actinide series]] of the [[periodic table]]. The heaviest naturally occurring element, uranium is nearly twice as dense as [[lead]] and weakly [[radioactivity|radioactive]]. It occurs naturally in low concentrations (a few parts per million) in soil, rock and water, and is commercially extracted from uranium-bearing [[mineral]]s such as [[uraninite]] (see [[uranium mining]]). | ||
| − | In nature, uranium atoms exist as [[uranium-238]] (99.275 | + | In nature, uranium atoms exist as [[uranium-238]] (99.275 percent), [[uranium-235]] (0.72 percent), and a very small amount of [[uranium-234]] (0.0058 percent). Uranium decays slowly by emitting an [[alpha particle]]. The [[half-life]] of uranium-238 is about 4.5 billion years and that of uranium-235 is 700 million years, making them useful in dating the [[age of the Earth]]. Along with [[thorium]] and [[plutonium]], it is one of the three [[fission|fissile]] elements, meaning it can easily break apart to become lighter elements. This property of uranium-235 and to a lesser degree uranium-233 generates the heat needed to run [[nuclear reactor]]s and provides the explosive material for [[nuclear weapon]]s. Both uses rely on the ability of uranium to produce a sustained [[nuclear chain reaction]]. [[Depleted uranium]] (uranium-238) is used in [[kinetic energy penetrator]]s and [[Vehicle armour|armor plating]].<ref name="BuildingBlocks479">John Emsley. ''Nature's Building Blocks.'' (Oxford: Oxford Univ. Press, 2001), 479</ref> |
| − | + | {{toc}} | |
In addition to its uses in nuclear technology, uranium has been used as a colorant in [[uranium glass]], producing orange-red through lemon yellow hues. It was also used for tinting in early [[photography]]. | In addition to its uses in nuclear technology, uranium has been used as a colorant in [[uranium glass]], producing orange-red through lemon yellow hues. It was also used for tinting in early [[photography]]. | ||
| Line 67: | Line 67: | ||
[[Image:Pichblende.jpg|thumb|left|[[Uraninite]], also known as Pichblende, is the most common ore mined to extract uranium.]] | [[Image:Pichblende.jpg|thumb|left|[[Uraninite]], also known as Pichblende, is the most common ore mined to extract uranium.]] | ||
| − | Uranium is a naturally occurring element that can be found in low levels within all rock, soil, and water. Uranium is also the highest-numbered element to be found naturally in significant quantities on earth and is always found combined with other elements.<ref name="LANL"/> It, along with all elements with [[atomic weight]]s higher than [[iron]], are only naturally formed in [[supernova]] explosions.<ref> | + | Uranium is a naturally occurring element that can be found in low levels within all rock, soil, and water. Uranium is also the highest-numbered element to be found naturally in significant quantities on earth and is always found combined with other elements.<ref name="LANL"/> It, along with all elements with [[atomic weight]]s higher than [[iron]], are only naturally formed in [[supernova]] explosions.<ref>[http://www.nasa.gov/worldbook/supernova_worldbook_prt.htm WorldBook@NASA: Supernova] ''NASA''. accessdate 2007-02-19</ref> The decay of uranium, [[thorium]] and [[Potassium#Isotopes|potassium-40]] in the Earth's [[Earth's mantle|mantle]] is thought to be the main source of heat<ref>Celeste Biever, [http://www.newscientist.com/channel/earth/mg18725103.700 First measurements of Earth's core radioactivity] ''New Scientist'' 27 July 2005</ref><ref>[http://physicsweb.org/articles/news/7/5/4/1 Potassium-40 heats up Earth's core] ''physicsweb.org'' May 7, 2003. accessdate 2007-01-14</ref> that keeps the outer core liquid and drives [[mantle convection]], which in turn drives [[plate tectonics]]. |
| − | Its average concentration in the [[Earth]]'s crust is (depending on the reference) 2 to 4 parts per million,<ref name="SciTechEncy"> | + | Its average concentration in the [[Earth]]'s crust is (depending on the reference) 2 to 4 parts per million,<ref name="SciTechEncy">"Uranium." ''The McGraw-Hill Science and Technology Encyclopedia,'' 5th ed. (The McGraw-Hill Companies, Inc.) [http://www.answers.com/uranium]. Retrieved October 30, 2008.</ref><ref name="BuildingBlocks480"/> or about 40 times as abundant as [[silver]].<ref name="ColumbiaEncy"/> The Earth's crust from the surface to 25 km (15 miles) down is calculated to contain 10<sup>17</sup> kg (2 x 10<sup>17</sup> lb) of uranium while the [[ocean]]s may contain 10<sup>13</sup> kg (2 x 10<sup>13</sup> lb).<ref name="SciTechEncy"/> The concentration of uranium in soil ranges from 0.7 to 11 parts per million (up to 15 parts per million in farmland soil due to use of phosphate [[fertilizer]]s) and 3 parts per billion of sea water is composed of the element.<ref name="BuildingBlocks480"/> |
| − | It is more plentiful than [[antimony]], [[tin]], [[cadmium]], [[mercury (element)|mercury]], or silver and is about as abundant as [[arsenic]] or [[molybdenum]]. | + | It is more plentiful than [[antimony]], [[tin]], [[cadmium]], [[mercury (element)|mercury]], or silver and is about as abundant as [[arsenic]] or [[molybdenum]].<ref name="BuildingBlocks480"/> It is found in hundreds of minerals including [[uraninite]] (the most common uranium ore), [[autunite]], [[uranophane]], [[torbernite]], and [[coffinite]]. Significant concentrations of uranium occur in some substances such as [[phosphate]] rock deposits, and minerals such as [[lignite]], and [[monazite]] sands in uranium-rich [[ore]]s<ref name="LANL"/> (it is recovered commercially from these sources with as little as 0.1 percent uranium<ref name="ColumbiaEncy"/>). |
[[Image:Citrobacter freundii.jpg|thumb|''[[Citrobacter]]'' species can have concentrations of uranium in their bodies 300 times higher than in the surrounding environment]] | [[Image:Citrobacter freundii.jpg|thumb|''[[Citrobacter]]'' species can have concentrations of uranium in their bodies 300 times higher than in the surrounding environment]] | ||
| − | + | ||
It has been shown in some recent work at [[Manchester]] that [[bacteria]] can reduce and fix uranium in [[soil]]s. This research is continuing at the university of [[Plymouth]] by Dr Keith Roach and S Handley. | It has been shown in some recent work at [[Manchester]] that [[bacteria]] can reduce and fix uranium in [[soil]]s. This research is continuing at the university of [[Plymouth]] by Dr Keith Roach and S Handley. | ||
| − | + | Some [[micro-organism]]s, such as the lichen ''[[Trapelia involuta]]'' or the bacterium ''[[Citrobacter]],'' can absorb concentrations of uranium that are up to 300 times higher than their environment.<ref>Emsley, 476, 482</ref> ''Citrobactor'' species absorb uranyl ions when given [[glycerol phosphate]] (or other similar organic [[phosphate]]s). After one day, one gram of bacteria will encrust themselves with nine grams of uranyl phosphate crystals; creating the possibility that these organisms could be used to decontaminate uranium-polluted water.<ref name="BuildingBlocks477"/><ref>L. E. Macaskie, R. M. Empson, A. K. Cheetham, C. P. Grey, A. J. Skarnulis, Uranium bioaccumulation by a Citrobacter sp. as a result of enzymically mediated growth of polycrystalline HUO<sub>2</sub>PO<sub>4</sub>. ''Science'' 257 (1992): 782-784. doi = 10.1126/science.1496397</ref> | |
| − | Some [[micro-organism]]s, such as the lichen ''[[Trapelia involuta]]'' or the bacterium ''[[Citrobacter]]'' | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[Plant]]s absorb some uranium from the soil they are rooted in. Dry weight concentrations of uranium in plants range from 5 to 60 parts per billion and ash from burnt wood can have concentrations up to 4 parts per million.<ref name="BuildingBlocks477"/> Dry weight concentrations of uranium in [[food]] plants are typically lower with one to two micrograms per day ingested through the food people eat.<ref name="BuildingBlocks477"/> | + | [[Plant]]s absorb some uranium from the [[soil]] they are rooted in. Dry weight concentrations of uranium in plants range from 5 to 60 parts per billion and ash from burnt wood can have concentrations up to 4 parts per million.<ref name="BuildingBlocks477"/> Dry weight concentrations of uranium in [[food]] plants are typically lower with one to two micrograms per day ingested through the food people eat.<ref name="BuildingBlocks477"/> |
===Production and reserves=== | ===Production and reserves=== | ||
[[Image:Yellowcake.jpg|180px|left|thumb|[[Yellowcake]] is a concentrated mixture of uranium oxides that is further refined to extract pure uranium.]] | [[Image:Yellowcake.jpg|180px|left|thumb|[[Yellowcake]] is a concentrated mixture of uranium oxides that is further refined to extract pure uranium.]] | ||
| − | Uranium ore is mined in several ways: by [[open pit mining|open pit]], [[underground mining|underground]] or by leaching uranium from low-grade ores (see [[uranium mining]]).<ref name="BuildingBlocks479"/> Uranium ore typically contains 0.1 to 0.25 | + | Uranium ore is mined in several ways: by [[open pit mining|open pit]], [[underground mining|underground]] or by leaching uranium from low-grade ores (see [[uranium mining]]).<ref name="BuildingBlocks479"/> Uranium ore typically contains 0.1 to 0.25 percent of actual uranium oxides so extensive measures must be employed to extract the metal from its ore.<ref name="EncyChem774">Seaborg, ''Encyclopedia of the Chemical Elements'' (1968), 774</ref> Uranium ore is crushed and rendered into a fine powder and then leached with either an [[acid]] or [[alkali]]. The leachate is then subjected to one of several sequences of precipitation, solvent extraction, and ion exchange. The resulting mixture, called [[yellowcake]], contains at least 75 percent uranium oxides. Yellowcake is then generally further refined using [[nitric acid]] to create a solution of [[uranyl nitrate]]. Additional solvent extraction procedures finish the process.<ref name="EncyChem774"/> |
| − | Commercial-grade uranium can be produced through the [[redox|reduction]] of uranium [[halide]]s with [[alkali metals|alkali]] or [[alkaline earth metal]]s. | + | Commercial-grade uranium can be produced through the [[redox|reduction]] of uranium [[halide]]s with [[alkali metals|alkali]] or [[alkaline earth metal]]s. Uranium metal can also be made through [[electrolysis]] of [[potassium|K]]U[[fluorine|F]]<sub>5</sub> or UF<sub>4</sub>, dissolved in a molten [[calcium chloride]] (Ca[[chloride|Cl]]<sub>2</sub>) and [[sodium chloride]] ([[sodium|Na]]Cl). Very pure uranium can be produced through the [[thermal decomposition]] of uranium halides on a hot filament.<ref name="LANL"/> |
| − | [[Image:KarteUrangewinnung.png|thumb|right|10 countries are responsible for over 95 | + | [[Image:KarteUrangewinnung.png|thumb|right|10 countries are responsible for over 95 percent of the global production of concentrated uranium oxides.]] |
| − | In 2005 seventeen countries produced concentrated uranium oxides; with [[Canada]] (27.9 | + | In 2005 seventeen countries produced concentrated uranium oxides; with [[Canada]] (27.9 percent) and [[Australia]] (22.8 percent) being the largest producers and [[Kazakhstan]] (10.5 percent), [[Russia]] (8.0 percent), [[Namibia]] (7.5 percent), [[Niger]] (7.4 percent), [[Uzbekistan]] (5.5 percent), the [[United States]] (2.5 percent), the [[Ukraine]] (1.9 percent), and [[China]] (1.7 percent) also producing significant amounts.<ref> World Uranium Production. ''UxC Consulting Company, LLC'' </ref> Three million metric ton of uranium ore reserves are known to exist and an additional five billion metric ton of uranium are estimated to be in [[sea water]] ([[Japan]]ese scientists in the 1980s proved that extraction of uranium from sea water using [[ion exchange]]ers was feasible).<ref name="BuildingBlocks479"/> |
| − | Australia has the world's largest uranium ore | + | Australia has the world's largest uranium ore reserves—40 percent of the planet's known supply. In fact, the world's largest single uranium deposit is located at the [[Olympic Dam]] Mine in [[South Australia]].<ref> [http://www.uraniumsa.org/processing/processing.htm Uranium Mining and Processing in South Australia] ''South Australian Chamber of Mines and Energy'' 2002. accessdate 2007-01-14 </ref> Almost all the uranium is exported, but under strict [[International Atomic Energy Agency]] safeguards to satisfy the Australian people and government that none of the uranium is used in [[nuclear weapons]]. [[As of 2006]], the Australian government was advocating an expansion of uranium mining, although issues with state governments and indigenous interests complicate the issue.<ref>"Nuclear Balance of Power," ''BRW'', 26 Oct. 2006, 41–44</ref> |
| − | The largest single domestic source of uranium in the United states was the [[Colorado Plateau]] located in Colorado, Utah, New Mexico, and Arizona. United States Federal government paid discovery bonuses and guaranteed purchase prices to | + | The largest single domestic source of uranium in the United states was the [[Colorado Plateau]] located in Colorado, Utah, New Mexico, and Arizona. United States Federal government paid discovery bonuses and guaranteed purchase prices to anyone who found and delivered Uranium ore. The United States Government was the sole legal purchaser of the uranium. The economic incentives resulted in a frenzy of exploration and mining activity throughout the Colorado plateau from 1947 through 1959 that left thousands of miles of crudely graded roads spiderwebbing the remote deserts of the Colorado Plateau, and thousands of abandoned uranium mines, exploratory shafts, and tailings piles. The frenzy ended as suddenly as it had begun, when the U.S. governments stopped purchasing the uranium. |
== History == | == History == | ||
===Pre-discovery use=== | ===Pre-discovery use=== | ||
| − | The use of uranium, in its natural [[oxide]] form, dates back to at least 79 C.E., when it was used to add a yellow color to [[ceramic]] glazes.<ref name="LANL"> | + | The use of uranium, in its natural [[oxide]] form, dates back to at least 79 C.E., when it was used to add a yellow color to [[ceramic]] glazes.<ref name="LANL">[http://periodic.lanl.gov/elements/92.html Uranium]. ''Los Alamos National Laboratory'' accessdate 2007-01-14</ref> Yellow glass with 1 percent uranium oxide was found in a [[Roman Empire|Roman]] villa on Cape Posilipo in the [[Bay of Naples]], [[Italy]] by R. T. Gunther of the [[University of Oxford]] in 1912.<ref name="BuildingBlocks482">Emsley, 482</ref> Starting in the late [[Middle Ages]], [[pitchblende]] was extracted from the [[Habsburg]] silver mines in [[Joachimsthal]], [[Bohemia]] (now in the [[Czech Republic]]) and was used as a coloring agent in the local [[glassmaking]] industry.<ref name="BuildingBlocks477"/> In the early nineteenth century, the world's only known source of uranium ores were these old mines. |
===Discovery=== | ===Discovery=== | ||
[[Image:Becquerel plate.jpg|thumb|left|[[Antoine Becquerel]] discovered the phenomenon of [[radioactivity]] by exposing a photographic plate to uranium (1896).]] | [[Image:Becquerel plate.jpg|thumb|left|[[Antoine Becquerel]] discovered the phenomenon of [[radioactivity]] by exposing a photographic plate to uranium (1896).]] | ||
| − | The [[discovery of the chemical elements|discovery]] of the element is credited to the German pharmacist [[Martin Heinrich Klaproth]], who named the new element after the planet [[Uranus]]. While working in his experimental laboratory in [[Berlin]] in 1789, Klaproth was able to precipitate a yellow compound (likely [[sodium diuranate]]) by dissolving pitchblende in [[nitric acid]] and neutralizing the solution with [[sodium hydroxide]].<ref name="BuildingBlocks477">Emsley, | + | The [[discovery of the chemical elements|discovery]] of the element is credited to the German pharmacist [[Martin Heinrich Klaproth]], who named the new element after the planet [[Uranus]]. While working in his experimental laboratory in [[Berlin]] in 1789, Klaproth was able to precipitate a yellow compound (likely [[sodium diuranate]]) by dissolving pitchblende in [[nitric acid]] and neutralizing the solution with [[sodium hydroxide]].<ref name="BuildingBlocks477">Emsley, 477</ref> Klaproth mistakenly assumed the yellow substance was the oxide of a yet-undiscovered element and heated it with [[charcoal]] to obtain a black powder, which he thought was the newly discovered metal itself (in fact, that powder was an oxide of uranium).<ref name="BuildingBlocks477"/><ref>[[Martin Heinrich Klaproth|M. H. Klaproth]], Chemische Untersuchung des Uranits, einer neuentdeckten metallischen Substanz. ''Chemische Annalen'' 2 |
| − | + | (1789): 387-403</ref> He named the newly discovered element after the planet [[Uranus (planet)|Uranus]], which had been discovered eight years earlier by [[William Herschel]]. | |
| − | + | In 1841, Eugene-Melchior Peligot, who was Professor of Analytical Chemistry at the Central School of Arts and Manufactures in [[Paris]], isolated the first sample of uranium [[metal]] by heating [[uranium tetrachloride]] with [[potassium]].<ref>E.-M. Peligot, Recherches Sur L'Uranium. ''Annales de chimie et de physique'' 5 (5) (1842): 5-47 [http://gallica.bnf.fr/ark:/12148/bpt6k34746s/f4.table] </ref><ref name="BuildingBlocks477"/> Uranium was not seen as being particularly dangerous during much of the nineteenth century, leading to the development of various uses for the element. One such use for the oxide was the coloring of [[pottery]] and [[glass]]. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | In 1841, Eugene-Melchior Peligot, who was Professor of Analytical Chemistry at the Central School of Arts and Manufactures in [[Paris]], isolated the first sample of uranium [[metal]] by heating [[uranium tetrachloride]] with [[potassium]].<ref> | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Antoine Becquerel]] discovered [[radioactive decay|radioactivity]] by using uranium in 1896.<ref name="ColumbiaEncy"/> Becquerel made the discovery in Paris by leaving a sample of uranium on top of an unexposed [[photographic plate]] in a drawer and noting that the plate had become 'fogged'.<ref name="BuildingBlocks478"/> He determined that a form of invisible light or rays emitted by uranium had exposed the plate. | [[Antoine Becquerel]] discovered [[radioactive decay|radioactivity]] by using uranium in 1896.<ref name="ColumbiaEncy"/> Becquerel made the discovery in Paris by leaving a sample of uranium on top of an unexposed [[photographic plate]] in a drawer and noting that the plate had become 'fogged'.<ref name="BuildingBlocks478"/> He determined that a form of invisible light or rays emitted by uranium had exposed the plate. | ||
| Line 134: | Line 110: | ||
[[Image:ChicagoPileTeam.png|right|thumb|left|[[Enrico Fermi]] (bottom left) and the rest of the team that initiated the first artificial [[nuclear chain reaction]] (1942).]] | [[Image:ChicagoPileTeam.png|right|thumb|left|[[Enrico Fermi]] (bottom left) and the rest of the team that initiated the first artificial [[nuclear chain reaction]] (1942).]] | ||
| − | A team led by [[Enrico Fermi]] in 1934 observed that bombarding uranium with neutrons produces the emission of [[beta decay|beta rays]] ([[electron]]s or [[positron]]s; see [[beta particle]]).<ref name="EncyChem773"/> The experiments leading to the discovery of uranium's ability to [[Nuclear fission|fission]] (break apart) into lighter elements and release [[binding energy]] were conducted by [[Otto Hahn]] and [[Fritz Strassmann]]<ref name="EncyChem773">Seaborg, ''Encyclopedia of the Chemical Elements'' (1968), | + | A team led by [[Enrico Fermi]] in 1934 observed that bombarding uranium with neutrons produces the emission of [[beta decay|beta rays]] ([[electron]]s or [[positron]]s; see [[beta particle]]).<ref name="EncyChem773"/> The experiments leading to the discovery of uranium's ability to [[Nuclear fission|fission]] (break apart) into lighter elements and release [[binding energy]] were conducted by [[Otto Hahn]] and [[Fritz Strassmann]]<ref name="EncyChem773">Seaborg, ''Encyclopedia of the Chemical Elements'' (1968), 773</ref> in Hahn's laboratory in Berlin. Lise Meitner and her nephew, physicist [[Otto Robert Frisch]], published the physical explanation in February 1939 and named the process 'nuclear fission'.<ref>[[Lise Meitner|L. Meitner]], [[Otto Frisch|O. Frisch]]. Disintegration of Uranium by Neutrons: a New Type of Nuclear Reaction. ''Nature'' 143 (1939): 239-240. doi 10.1038/224466a0. [http://dbhs.wvusd.k12.ca.us/webdocs/Chem-History/Meitner-Fission-1939.html]</ref> Soon after, Fermi hypothesized that the fission of uranium might release enough neutrons to sustain a fission reaction. Confirmation of this hypothesis came in 1939 and later work found that 2 1/2 neutrons are released by each fission of the rare uranium isotope [[uranium-235]].<ref name="EncyChem773"/> Further work found that the far more common [[uranium-238]] isotope can be [[transmutation|transmuted]] into [[plutonium]], which, like uranium-235, is also fissionable by thermal neutrons. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | On December 2 1942, another team led by Enrico Fermi was able to initiate the first artificial [[nuclear chain reaction]]. Working in a lab below the stands of Stagg Field at the [[University of Chicago]], the team created the conditions needed for such a reaction by piling together 400 tons (360 metric tons) of [[graphite]], 58 tons (53 metric tons) of [[uranium oxide]], and six tons (five and a half metric tons) of uranium metal.<ref name="EncyChem773"/> Later researchers found that such a chain reaction could either be controlled to produce usable energy or could be allowed to go out of control to produce an explosion more violent than anything possible using [[Explosive material|chemical explosives]]. | + | On December 2, 1942, another team led by Enrico Fermi was able to initiate the first artificial [[nuclear chain reaction]]. Working in a lab below the stands of Stagg Field at the [[University of Chicago]], the team created the conditions needed for such a reaction by piling together 400 tons (360 metric tons) of [[graphite]], 58 tons (53 metric tons) of [[uranium oxide]], and six tons (five and a half metric tons) of uranium metal.<ref name="EncyChem773"/> Later researchers found that such a chain reaction could either be controlled to produce usable energy or could be allowed to go out of control to produce an explosion more violent than anything possible using [[Explosive material|chemical explosives]]. |
===Bombs and reactors=== | ===Bombs and reactors=== | ||
[[Image:Atomic cloud over Hiroshima.jpg|thumb|left|The [[mushroom cloud]] over [[Hiroshima]] after the dropping of the uranium-based atomic bomb nicknamed '[[Little Boy]]' (1945).]] | [[Image:Atomic cloud over Hiroshima.jpg|thumb|left|The [[mushroom cloud]] over [[Hiroshima]] after the dropping of the uranium-based atomic bomb nicknamed '[[Little Boy]]' (1945).]] | ||
| − | Two major types of atomic bomb were developed in the [[Manhattan Project]] during [[World War II]]: a [[plutonium]]-based device (see [[Trinity test]] and '[[Fat Man]]') whose plutonium was derived from uranium-238, and a uranium-based device (nicknamed '[[Little Boy]]') whose fissile material was highly [[enriched uranium]]. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the [[Japan]]ese city of [[Hiroshima]] on August 6 1945. Exploding with a yield equivalent to 12,500 metric tons of [[Trinitrotoluene|TNT]], the blast and thermal wave of the bomb destroyed nearly 50,000 buildings and killed approximately 75,000 people (see [[Atomic bombings of Hiroshima and Nagasaki]]).<ref name="BuildingBlocks478"/> | + | Two major types of atomic bomb were developed in the [[Manhattan Project]] during [[World War II]]: a [[plutonium]]-based device (see [[Trinity test]] and '[[Fat Man]]') whose plutonium was derived from uranium-238, and a uranium-based device (nicknamed '[[Little Boy]]') whose fissile material was highly [[enriched uranium]]. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the [[Japan]]ese city of [[Hiroshima]] on August 6, 1945. Exploding with a yield equivalent to 12,500 metric tons of [[Trinitrotoluene|TNT]], the blast and thermal wave of the bomb destroyed nearly 50,000 buildings and killed approximately 75,000 people (see [[Atomic bombings of Hiroshima and Nagasaki]]).<ref name="BuildingBlocks478"/> Initially it was believed that uranium was relatively rare, and that [[nuclear proliferation]] could be avoided by simply buying up all known uranium stocks, but within a decade large deposits of it were discovered in many places around the world. |
[[Image:First four nuclear lit bulbs.jpeg|thumb|Four light bulbs lit with electricity generated from the first artificial [[nuclear reactor]], [[Experimental Breeder Reactor I|EBR-I]] (1951).]] | [[Image:First four nuclear lit bulbs.jpeg|thumb|Four light bulbs lit with electricity generated from the first artificial [[nuclear reactor]], [[Experimental Breeder Reactor I|EBR-I]] (1951).]] | ||
| − | [[Experimental Breeder Reactor I]] at the [[Idaho National Laboratory|Idaho National Engineering and Environmental Laboratory]] near [[Arco, Idaho]] became the first functioning artificial nuclear reactor on December 20 1951. Initially, only four 150-watt light bulbs were lit by the reactor but improvements eventually enabled it to power the whole facility (later, the whole town of Arco became the first in the world to have all its [[electricity]] come from nuclear power).<ref> | + | [[Experimental Breeder Reactor I]] at the [[Idaho National Laboratory|Idaho National Engineering and Environmental Laboratory]] near [[Arco, Idaho]] became the first functioning artificial nuclear reactor on December 20 1951. Initially, only four 150-watt light bulbs were lit by the reactor but improvements eventually enabled it to power the whole facility (later, the whole town of Arco became the first in the world to have all its [[electricity]] come from nuclear power).<ref>[http://web.em.doe.gov/tie/history.html History and Success of Argonne National Laboratory: Part 1]. ''U.S. Department of Energy, Argonne National Laboratory'' 1998. accessdate 2007-01-28</ref> The world's first commercial scale nuclear power station, [[Sellafield|Calder Hall]], in England, began generation on October 17 1956.<ref name="BBC"> ''BBC News, On this Day'' [http://news.bbc.co.uk/onthisday/hi/dates/stories/october/17/newsid_3147000/3147145.stm 1956: Queen switches on nuclear power] accessdate June 28, 2006</ref> Another early power reactor was the [[Shippingport Reactor]] in [[Pennsylvania]], which began electricity production in 1957. Nuclear power was used for the first time for propulsion by a [[submarine]], the [[USS Nautilus (SSN-571)|USS ''Nautilus'']], in 1954.<ref name="EncyChem773"/> |
| − | Fifteen ancient and no longer active natural fission reactors were found in three separate ore deposits at the [[Oklo]] mine in [[Gabon]], [[West Africa]] in 1972. Discovered by French physicist [[Francis Perrin]], they are collectively known as the [[Natural nuclear fission reactor|Oklo Fossil Reactors]]. The ore they exist in is 1.7 billion years old; at that time, uranium-235 comprised about three percent of the total uranium on Earth.<ref name="OCRWM"> | + | Fifteen ancient and no longer active natural fission reactors were found in three separate ore deposits at the [[Oklo]] mine in [[Gabon]], [[West Africa]] in 1972. Discovered by French physicist [[Francis Perrin]], they are collectively known as the [[Natural nuclear fission reactor|Oklo Fossil Reactors]]. The ore they exist in is 1.7 billion years old; at that time, uranium-235 comprised about three percent of the total uranium on Earth.<ref name="OCRWM">[http://www.ocrwm.doe.gov/factsheets/doeymp0010.shtml Oklo: Natural Nuclear Reactors] ''Office of Civilian Radioactive Waste Management'' accessdate June 28 2006</ref> This is high enough to permit nuclear fission to occur, providing other conditions are right. The ability of the surrounding sediment to contain the [[nuclear waste]] products in less than ideal conditions has been cited by the U.S. federal government as evidence of their claim that the [[Yucca Mountain]] facility could safely be a repository of waste for the [[nuclear power]] industry.<ref name="OCRWM"/> |
===Cold War legacy and waste=== | ===Cold War legacy and waste=== | ||
| Line 165: | Line 132: | ||
Since the [[break-up of the Soviet Union]] in 1991, an estimated 600 tons (540 metric tons) of highly-enriched weapons grade uranium (enough to make 40,000 nuclear warheads) have been stored in often inadequately guarded facilities in the [[Russia|Russian Federation]] and several other former Soviet states.<ref name="EncyIntel"/> Police in [[Asia]], [[Europe]], and [[South America]] on at least 16 occasions from 1993 to 2005 have intercepted shipments of smuggled bomb-grade uranium or plutonium, most of which was from ex-Soviet sources.<ref name="EncyIntel"/> From 1993 to 2005 the [[Material Protection, Control, and Accounting Program]], operated by the [[federal government of the United States]], spent approximately US$550 million to help safeguard uranium and plutonium stockpiles in Russia.<ref name="EncyIntel"/> | Since the [[break-up of the Soviet Union]] in 1991, an estimated 600 tons (540 metric tons) of highly-enriched weapons grade uranium (enough to make 40,000 nuclear warheads) have been stored in often inadequately guarded facilities in the [[Russia|Russian Federation]] and several other former Soviet states.<ref name="EncyIntel"/> Police in [[Asia]], [[Europe]], and [[South America]] on at least 16 occasions from 1993 to 2005 have intercepted shipments of smuggled bomb-grade uranium or plutonium, most of which was from ex-Soviet sources.<ref name="EncyIntel"/> From 1993 to 2005 the [[Material Protection, Control, and Accounting Program]], operated by the [[federal government of the United States]], spent approximately US$550 million to help safeguard uranium and plutonium stockpiles in Russia.<ref name="EncyIntel"/> | ||
| − | [[Nuclear fallout]] and pollution have occurred from above-ground [[nuclear test]]s<ref> | + | [[Nuclear fallout]] and pollution have occurred from above-ground [[nuclear test]]s<ref>T. Warneke, I. W. Croudace, P. E. Warwick, R. N. Taylor. A new ground-level fallout record of uranium and plutonium isotopes for northern temperate latitudes. ''Earth and Planetary Science Letters'' 2002 203 (3-4):1047-1057. doi = 10.1016/S0012-821X(02)00930-5 |
| − | + | </ref> and several [[nuclear accident]]s: the [[Windscale fire]] at the [[Sellafield]] nuclear plant in 1957 spread [[iodine-131]] over much of [[Northern England]], the [[Three Mile Island accident]] in 1979 released [[radon]] gas and some iodine-131, the [[Chernobyl disaster]] in 1986 released radon, iodine-131 and [[strontium-90]] that spread over much of [[Europe]].<ref name="BuildingBlocks480"/> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== Notable characteristics == | == Notable characteristics == | ||
[[Image:Nuclear_fission.svg.png|thumb|150px|An induced nuclear fission event involving uranium-235.]] | [[Image:Nuclear_fission.svg.png|thumb|150px|An induced nuclear fission event involving uranium-235.]] | ||
| − | Uranium is an [[inner transition metal]] of the actinide series, situated in period 7 of the [[periodic table]], between [[protactinium]] and [[neptunium]]. When refined, it is a silvery white, weakly radioactive [[metal]], which is slightly softer than [[steel]],<ref name="LANL" | + | Uranium is an [[inner transition metal]] of the actinide series, situated in period 7 of the [[periodic table]], between [[protactinium]] and [[neptunium]]. When refined, it is a silvery white, weakly radioactive [[metal]], which is slightly softer than [[steel]],<ref name="LANL"/> strongly [[electropositive]] and a poor [[electrical conductivity|electrical conductor]].<ref name="SciTechEncy"/> It is [[malleable]], [[ductile]], and slightly [[paramagnetic]].<ref name="LANL"/> Uranium metal has very high [[density]], 65 percent more dense than [[lead]], but slightly less dense than [[gold]]. |
| − | Uranium metal reacts with nearly all nonmetallic elements and their compounds with reactivity increasing with temperature.<ref name="ColumbiaEncy"> | + | Uranium metal reacts with nearly all nonmetallic elements and their compounds with reactivity increasing with temperature.<ref name="ColumbiaEncy">"uranium." ''Columbia Electronic Encyclopedia,'' 6th Ed. ''Columbia University Press'' </ref> [[Hydrochloric acid|Hydrochloric]] and [[nitric acid]]s dissolve uranium but nonoxidizing acids attack the element very slowly.<ref name="SciTechEncy"/> When finely divided, it can react with cold water; in air, uranium metal becomes coated with a dark layer of uranium oxide.<ref name="LANL"/> Uranium in ores is extracted chemically and converted into [[uranium dioxide]] or other chemical forms usable in industry. |
| − | Uranium was the first element that was found to be [[nuclear fission|fissile]]. Upon bombardment with slow [[neutron]]s, its uranium-235 [[isotope]] becomes a very short lived uranium-236 [[isomer]] which immediately divides into two smaller nuclei, releasing [[nuclear binding energy]] and more neutrons. If these neutrons are absorbed by other uranium-235 nuclei, a [[nuclear chain reaction]] occurs and, if there is nothing to absorb some neutrons and slow the reaction, the reaction is explosive. As little as 15 lb (7 kg) of uranium-235 can be used to make an atomic bomb.<ref name="EncyIntel"> | + | Uranium was the first element that was found to be [[nuclear fission|fissile]]. Upon bombardment with slow [[neutron]]s, its uranium-235 [[isotope]] becomes a very short lived uranium-236 [[isomer]] which immediately divides into two smaller nuclei, releasing [[nuclear binding energy]] and more neutrons. If these neutrons are absorbed by other uranium-235 nuclei, a [[nuclear chain reaction]] occurs and, if there is nothing to absorb some neutrons and slow the reaction, the reaction is explosive. As little as 15 lb (7 kg) of uranium-235 can be used to make an atomic bomb.<ref name="EncyIntel">''Encyclopedia of Espionage, Intelligence, and Security.'' The Gale Group, Inc. uranium [http://www.answers.com/uranium]</ref> The first atomic bomb worked by this principle (nuclear fission). |
Uranium metal has three [[allotrope|allotropic]] forms: | Uranium metal has three [[allotrope|allotropic]] forms: | ||
| Line 195: | Line 154: | ||
====Natural concentrations==== | ====Natural concentrations==== | ||
| − | Naturally occurring uranium is composed of three major [[isotope]]s, [[uranium-238]] (99.28 | + | Naturally occurring uranium is composed of three major [[isotope]]s, [[uranium-238]] (99.28 percent [[natural abundance]]), [[uranium-235]] (0.71 percent), and [[uranium-234]] (0.0054 percent). All three isotopes are [[radioactive]], creating [[radioisotope]]s, with the most abundant and stable being uranium-238 with a [[half-life]] of 4.51 × 10<sup>9</sup> years (close to the [[age of the Earth]]), uranium-235 with a half-life of 7.13 × 10<sup>8</sup> years, and uranium-234 with a half-life of 2.48 × 10<sup>5</sup> years.<ref name="EncyChem777">Seaborg, 777</ref> |
Uranium-238 is an α emitter, [[Radioactive Decay|decaying]] through the 18-member uranium natural decay series into [[lead#Isotopes|lead-206]].<ref name="ColumbiaEncy"/> The decay series of uranium-235 (also called actinouranium) has 15 members that ends in lead-207, [[protactinium#Isotopes|protactinium-231]] and [[actinium#isotopes|actinium-227]].<ref name="ColumbiaEncy"/> The constant rates of decay in these series makes comparison of the ratios of parent to daughter elements useful in [[radiometric dating]]. Uranium-233 is made from [[thorium#isotopes|thorium-232]] by [[neutron]] bombardment.<ref name="LANL"/> | Uranium-238 is an α emitter, [[Radioactive Decay|decaying]] through the 18-member uranium natural decay series into [[lead#Isotopes|lead-206]].<ref name="ColumbiaEncy"/> The decay series of uranium-235 (also called actinouranium) has 15 members that ends in lead-207, [[protactinium#Isotopes|protactinium-231]] and [[actinium#isotopes|actinium-227]].<ref name="ColumbiaEncy"/> The constant rates of decay in these series makes comparison of the ratios of parent to daughter elements useful in [[radiometric dating]]. Uranium-233 is made from [[thorium#isotopes|thorium-232]] by [[neutron]] bombardment.<ref name="LANL"/> | ||
| Line 206: | Line 165: | ||
Enrichment of uranium ore through [[isotope separation]] to concentrate the fissionable uranium-235 is needed for use in nuclear power plants and nuclear weapons. A majority of [[neutron]]s released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the [[nuclear chain reaction]] needed for these applications. The concentration and amount of uranium-235 needed to achieve this is called a 'critical mass.' | Enrichment of uranium ore through [[isotope separation]] to concentrate the fissionable uranium-235 is needed for use in nuclear power plants and nuclear weapons. A majority of [[neutron]]s released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the [[nuclear chain reaction]] needed for these applications. The concentration and amount of uranium-235 needed to achieve this is called a 'critical mass.' | ||
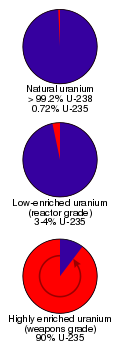
| − | To be considered 'enriched' the uranium-235 fraction has to be increased to significantly greater than its concentration in naturally-occurring uranium. Enriched uranium typically has a uranium-235 concentration of between 3 and 5 | + | To be considered 'enriched' the uranium-235 fraction has to be increased to significantly greater than its concentration in naturally-occurring uranium. Enriched uranium typically has a uranium-235 concentration of between 3 and 5 percent.<ref>[http://web.ead.anl.gov/uranium/guide/depletedu/enrich/index.cfm Uranium Enrichment] ''Argonne National Laboratory''. accessdate 2007-02-11 </ref> The process produces huge quantities of uranium that is depleted of uranium-235 and with a correspondingly increased fraction of uranium-238, called [[depleted uranium]] or 'DU'. To be considered 'depleted', the uranium-235 isotope concentration has to have been decreased to significantly less than its natural concentration. |
| − | The [[gas centrifuge]] process, where gaseous [[uranium hexafluoride]] (UF<sub>6</sub>) is separated by weight using high-speed [[centrifuge]]s, has become the cheapest and leading enrichment process (lighter UF<sub>6</sub> concentrates in the center of the centrifuge).<ref name="BuildingBlocks478">Emsley, | + | The [[gas centrifuge]] process, where gaseous [[uranium hexafluoride]] (UF<sub>6</sub>) is separated by weight using high-speed [[centrifuge]]s, has become the cheapest and leading enrichment process (lighter UF<sub>6</sub> concentrates in the center of the centrifuge).<ref name="BuildingBlocks478">Emsley, 478</ref> The [[gaseous diffusion]] process was the previous leading method for enrichment and the one used in the [[Manhattan Project]]. In this process, uranium hexafluoride is repeatedly [[diffusion|diffused]] through a [[silver]]-[[zinc]] membrane and the different isotopes of uranium are separated by diffusion rate (uranium 238 is heavier and thus diffuses slightly slower than uranium-235).<ref name="BuildingBlocks478"/> The [[laser excitation]] method employs a [[laser]] beam of precise energy to sever the bond between uranium-235 and fluorine. This leaves uranium-238 bonded to fluorine and allows uranium-235 metal to precipitate from the solution.<ref name="BuildingBlocks479"/> Another method is called [[liquid thermal diffusion]].<ref name="SciTechEncy"/> |
== Compounds == | == Compounds == | ||
| Line 214: | Line 173: | ||
[[Image:U3O8lattice.jpg|thumb|[[Triuranium octaoxide]] (diagram pictured) and [[uranium dioxide]] are the two most common uranium oxides]] | [[Image:U3O8lattice.jpg|thumb|[[Triuranium octaoxide]] (diagram pictured) and [[uranium dioxide]] are the two most common uranium oxides]] | ||
| − | [[Ion]]s that represent the four different [[oxidation state]]s of uranium are [[solubility|soluble]] and therefore can be studied in [[aqueous solution]]s. They are: U<sup>3+</sup> (red), U<sup>4+</sup> (green), U[[oxygen|O]]<sub>2</sub><sup>+</sup> (unstable), and UO<sup>2+</sup> (yellow).<ref name="EncyChem778">Seaborg, ''Encyclopedia of the Chemical Elements'' (1968), | + | [[Ion]]s that represent the four different [[oxidation state]]s of uranium are [[solubility|soluble]] and therefore can be studied in [[aqueous solution]]s. They are: U<sup>3+</sup> (red), U<sup>4+</sup> (green), U[[oxygen|O]]<sub>2</sub><sup>+</sup> (unstable), and UO<sup>2+</sup> (yellow).<ref name="EncyChem778">Seaborg, ''Encyclopedia of the Chemical Elements'' (1968), 778</ref> A few [[solid]] and semi-metallic [[compound (chemistry)|compound]]s such as UO and US exist for the formal oxidation state uranium(II) but no simple ions are known to exist in solution for that state. Ions of U<sup>3+</sup>liberate [[hydrogen]] from [[water]] and are therefore considered to be highly unstable. The UO<sub>2</sub><sup>+</sup> ion represents the uranium(V) state and is known to form compounds that include inorganic ions such as [[carbonate]], [[chloride]] and [[sulfate]], and various organic [[chelating]] agents.<ref name="EncyChem778"/> |
| − | [[phase (matter)|Phase relationship]]s in the uranium-[[oxygen]] system are highly complex. The most important oxidation states of uranium are uranium(IV) and uranium(VI) and their two corresponding [[oxide]]s are, respectively, [[uranium dioxide]] (UO<sub>2</sub>) and [[uranium trioxide]] (UO<sub>3</sub>).<ref name="EncyChem779">Seaborg, | + | [[phase (matter)|Phase relationship]]s in the uranium-[[oxygen]] system are highly complex. The most important oxidation states of uranium are uranium(IV) and uranium(VI) and their two corresponding [[oxide]]s are, respectively, [[uranium dioxide]] (UO<sub>2</sub>) and [[uranium trioxide]] (UO<sub>3</sub>).<ref name="EncyChem779">Seaborg, 779</ref> Other [[uranium oxide]]s, such as uranium monoxide (UO), diuranium pentoxide (U<sub>2</sub>O<sub>5</sub>), and uranium peroxide (UO<sub>4</sub>•2H<sub>2</sub>O) are also known to exist. |
| − | The most common forms of uranium oxide are [[triuranium octaoxide]] (U<sub>3</sub>O<sub>8</sub>) and the aforementioned UO<sub>2</sub>.<ref name="ANL-Chem"> | + | The most common forms of uranium oxide are [[triuranium octaoxide]] (U<sub>3</sub>O<sub>8</sub>) and the aforementioned UO<sub>2</sub>.<ref name="ANL-Chem">[http://web.ead.anl.gov/uranium/guide/ucompound/forms/index.cfm Chemical Forms of Uranium] ''Argonne National Laboratory''. accessdate 2007-02-18 </ref> Both oxide forms are solids that have low solubility in water and are relatively stable over a wide range of environmental conditions. Triuranium octaoxide is (depending on conditions) the most stable compound of uranium and is the form most commonly found in nature. Uranium dioxide is the form in which uranium is most commonly used as a nuclear reactor fuel.<ref name="ANL-Chem"/> At ambient temperatures, UO<sub>2</sub> will gradually convert to U<sub>3</sub>O<sub>8</sub>. Because of their stability, uranium oxides are generally considered the preferred chemical form for storage or disposal.<ref name="ANL-Chem"/> |
===Hydrides, carbides and nitrides=== | ===Hydrides, carbides and nitrides=== | ||
| − | Uranium metal heated to 250 to 300 °C reacts with [[hydrogen]] to form [[uranium hydride]]. Yet higher temperatures will reversibly remove the hydrogen. This property makes uranium hydrides convenient starting materials to create reactive uranium powder along with various uranium [[carbide]], [[nitride]], and [[halide]] compounds.<ref name="EncyChem782">Seaborg, | + | Uranium metal heated to 250 to 300 °C reacts with [[hydrogen]] to form [[uranium hydride]]. Yet higher temperatures will reversibly remove the hydrogen. This property makes uranium hydrides convenient starting materials to create reactive uranium powder along with various uranium [[carbide]], [[nitride]], and [[halide]] compounds.<ref name="EncyChem782">Seaborg, 782</ref> Two crystal modifications of uranium hydride exist: an α form that is obtained at low temperatures and a β form that is created when the formation temperature is above 250 °C.<ref name="EncyChem782"/> |
| − | [[Uranium carbide]]s and [[uranium nitride]]s are both relatively [[inert]] [[semimetal]]lic compounds that are minimally soluble in [[acid]]s, react with [[water]], and can ignite in [[air]] to form U<sub>3</sub>O<sub>8</sub>.<ref name="EncyChem782"/> Carbides of uranium include uranium monocarbide (U[[carbon|C]]), uranium dicarbide (UC<sub>2</sub>), and diuranium tricarbide (U<sub>2</sub>C<sub>3</sub>). Both UC and UC<sub>2</sub> are formed by adding carbon to molten uranium or by exposing the metal to [[carbon monoxide]] at high temperatures. Stable below 1800 °C, U<sub>2</sub>C<sub>3</sub> is prepared by subjecting a heated mixture of UC and UC<sub>2</sub> to mechanical stress.<ref name="EncyChem780">Seaborg, | + | [[Uranium carbide]]s and [[uranium nitride]]s are both relatively [[inert]] [[semimetal]]lic compounds that are minimally soluble in [[acid]]s, react with [[water]], and can ignite in [[air]] to form U<sub>3</sub>O<sub>8</sub>.<ref name="EncyChem782"/> Carbides of uranium include uranium monocarbide (U[[carbon|C]]), uranium dicarbide (UC<sub>2</sub>), and diuranium tricarbide (U<sub>2</sub>C<sub>3</sub>). Both UC and UC<sub>2</sub> are formed by adding carbon to molten uranium or by exposing the metal to [[carbon monoxide]] at high temperatures. Stable below 1800 °C, U<sub>2</sub>C<sub>3</sub> is prepared by subjecting a heated mixture of UC and UC<sub>2</sub> to mechanical stress.<ref name="EncyChem780">Seaborg, 780</ref> Uranium nitrides obtained by direct exposure of the metal to [[nitrogen]] include uranium mononitride (UN), uranium dinitride (UN<sub>2</sub>), and diuranium trinitride (U<sub>2</sub>N<sub>3</sub>).<ref name="EncyChem780"/> |
===Halides=== | ===Halides=== | ||
| Line 248: | Line 207: | ||
The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of [[depleted uranium]] (DU) alloyed with 1–2% other elements. At high impact speed, the density, hardness, and flammability of the projectile enable destruction of heavily armored targets. Tank armor and the removable armor on combat vehicles are also hardened with depleted uranium (DU) plates. The use of DU became a contentious political-environmental issue after U.S., UK and other countries' use of DU munitions in wars in the Persian Gulf and the Balkans raised questions of uranium compounds left in the soil (see [[Gulf War Syndrome]]).<ref name="EncyIntel"/> | The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of [[depleted uranium]] (DU) alloyed with 1–2% other elements. At high impact speed, the density, hardness, and flammability of the projectile enable destruction of heavily armored targets. Tank armor and the removable armor on combat vehicles are also hardened with depleted uranium (DU) plates. The use of DU became a contentious political-environmental issue after U.S., UK and other countries' use of DU munitions in wars in the Persian Gulf and the Balkans raised questions of uranium compounds left in the soil (see [[Gulf War Syndrome]]).<ref name="EncyIntel"/> | ||
| − | Depleted uranium is also used as a shielding material in some containers used to store and transport radioactive materials.<ref name="SciTechEncy"/> Other uses of DU include counterweights for aircraft control surfaces, as ballast for missile [[re-entry vehicle]]s and as a shielding material.<ref name="LANL"/> Due to its high density, this material is found in [[inertial guidance]] devices and in [[gyroscope|gyroscopic]] [[compass]]es.<ref name="LANL"/> DU is preferred over similarly dense metals due to its ability to be easily machined and cast.<ref name="BuildingBlocks480">Emsley, | + | Depleted uranium is also used as a shielding material in some containers used to store and transport radioactive materials.<ref name="SciTechEncy"/> Other uses of DU include counterweights for aircraft control surfaces, as ballast for missile [[re-entry vehicle]]s and as a shielding material.<ref name="LANL"/> Due to its high density, this material is found in [[inertial guidance]] devices and in [[gyroscope|gyroscopic]] [[compass]]es.<ref name="LANL"/> DU is preferred over similarly dense metals due to its ability to be easily machined and cast.<ref name="BuildingBlocks480">Emsley, 480</ref> |
| − | During the later stages of [[World War II]], the entire [[Cold War]] and to a much lesser extent afterwards, uranium was used as the fissile explosive material to produce [[nuclear weapon]]s. Two major types of fission bombs were built: a relatively simple device that uses [[uranium-235]] and a more complicated mechanism that uses [[uranium-238]]-derived [[plutonium-239]]. Later, a much more complicated and far more powerful fusion bomb that uses a plutonium-based device in a uranium casing to cause a mixture of [[tritium]] and [[deuterium]] to undergo [[nuclear fusion]] was built.<ref> | + | During the later stages of [[World War II]], the entire [[Cold War]] and to a much lesser extent afterwards, uranium was used as the fissile explosive material to produce [[nuclear weapon]]s. Two major types of fission bombs were built: a relatively simple device that uses [[uranium-235]] and a more complicated mechanism that uses [[uranium-238]]-derived [[plutonium-239]]. Later, a much more complicated and far more powerful fusion bomb that uses a plutonium-based device in a uranium casing to cause a mixture of [[tritium]] and [[deuterium]] to undergo [[nuclear fusion]] was built.<ref>[http://www.fas.org/nuke/intro/nuke/design.htm Nuclear Weapon Design] ''Federation of American Scientists'' 1998. accessdate 2007-02-19</ref> |
===Civilian=== | ===Civilian=== | ||
| Line 265: | Line 224: | ||
== Precautions == | == Precautions == | ||
===Exposure=== | ===Exposure=== | ||
| − | A person can be exposed to uranium (or its radioactive daughters such as [[radon]]) by inhaling dust in air or by ingesting contaminated water and food. The amount of uranium in air is usually very small; however, people who work in factories that process [[phosphate]] [[fertilizer]]s, live near government facilities that made or tested nuclear weapons, or live or work near a [[coal]]-fired power plant, facilities that mine or process uranium ore, or enrich uranium for reactor fuel, may have increased exposure to uranium.<ref name="EPA-Rad"> | + | A person can be exposed to uranium (or its radioactive daughters such as [[radon]]) by inhaling dust in air or by ingesting contaminated water and food. The amount of uranium in air is usually very small; however, people who work in factories that process [[phosphate]] [[fertilizer]]s, live near government facilities that made or tested nuclear weapons, or live or work near a [[coal]]-fired power plant, facilities that mine or process uranium ore, or enrich uranium for reactor fuel, may have increased exposure to uranium.<ref name="EPA-Rad">[http://www.epa.gov/radiation/radionuclides/uranium.htm Radiation Information for Uranium] ''U.S. Environmental Protection Agency'' accessdate 2007-02-18 </ref><ref name="ATSDR-ToxFAQ">[http://www.atsdr.cdc.gov/tfacts150.html ToxFAQ for Uranium] ''Agency for Toxic Substances and Disease Registry'' September 1999. accessdate 2007-02-18</ref> Houses or structures which are over uranium deposits (either natural or man-made slag deposits) may have an increased incidence of exposure to [[radon]] gas. |
| − | Almost all uranium that is ingested is excreted during [[digestion]], but up to 5 | + | Almost all uranium that is ingested is excreted during [[digestion]], but up to 5 percent is absorbed by the body when the soluble uranyl ion is ingested while only 0.5 percent is absorbed when insoluble forms of uranium, such as its oxide, are ingested.<ref name="BuildingBlocks477"/> However, soluble uranium compounds tend to quickly pass through the body whereas insoluble uranium compounds, especially when ingested via dust into the [[lung]]s, pose a more serious exposure hazard. After entering the bloodstream, the absorbed uranium tends to [[bioaccumulate]] and stay for many years in [[bone]] tissue because of uranium's affinity for phosphates.<ref name="BuildingBlocks477"/> Uranium does not absorb through the [[skin]], and [[alpha particle]]s released by uranium cannot penetrate the skin. |
===Effects=== | ===Effects=== | ||
| − | The greatest health risk from large intakes of uranium is toxic damage to the [[kidney]]s, because, in addition to being weakly radioactive, uranium is a toxic metal.<ref name="ATSDR"> | + | The greatest health risk from large intakes of uranium is toxic damage to the [[kidney]]s, because, in addition to being weakly radioactive, uranium is a toxic metal.<ref name="ATSDR">Toxicological Profile for Uranium [http://www.atsdr.cdc.gov/toxprofiles/tp150-c2.pdf] ''Agency for Toxic Substances and Disease Registry (ATSDR)'' Atlanta, GA. CAS# 7440-61-1 September 1999</ref><ref name="BuildingBlocks477"/> Radiological effects are generally local because this is the nature of alpha radiation, the primary form from U-238 decay. <!-- NEEDS CITE Uranium compounds in general are poorly absorbed by the lining in the lungs and may remain a radiological hazard indefinitely. Uranyl (UO<sub>2</sub><sup>+</sup>) ions, such as from [[uranium trioxide]] or uranyl nitrate and other hexavalent uranium compounds have been shown to cause birth defects and immune system damage in laboratory animals. /NEEDS CITE —> No human [[cancer]] of any type has ever been seen as a result of exposure to natural or depleted uranium<ref name="ATSDR-PHS">[http://www.atsdr.cdc.gov/toxprofiles/phs150.html Public Health Statement for Uranium] ''CDC'' accessdate 2007-02-15</ref> but exposure to some of its decay products, especially [[radon]], [[strontium-90]], and [[iodine-131]] does pose a significant health threat.<ref name="BuildingBlocks480"/> |
Although accidental inhalation exposure to a high concentration of uranium hexafluoride has | Although accidental inhalation exposure to a high concentration of uranium hexafluoride has | ||
| Line 287: | Line 246: | ||
==Notes== | ==Notes== | ||
| − | + | <references/> | |
==References== | ==References== | ||
| − | * Emsley, John. 2001. ''Nature's Building Blocks: An A–Z Guide to the Elements'' | + | * Emsley, John. 2001. ''Nature's Building Blocks: An A–Z Guide to the Elements.'' Oxford: Oxford Univ. Press. ISBN 0198503407. |
| − | * Greenwood, N.N., and A. Earnshaw. 1998. ''Chemistry of the Elements'' 2nd ed. Oxford, UK; Burlington, MA: Butterworth-Heinemann. ISBN 0750633654. [http://www.knovel.com/knovel2/Toc.jsp?BookID=402&VerticalID=0 Online version]. | + | * Greenwood, N.N., and A. Earnshaw. 1998. ''Chemistry of the Elements,'' 2nd ed. Oxford, UK; Burlington, MA: Butterworth-Heinemann. ISBN 0750633654. [http://www.knovel.com/knovel2/Toc.jsp?BookID=402&VerticalID=0 Online version]. |
| − | * Hampel, Clifford A. 1968. ''The Encyclopedia of the Chemical Elements'' | + | * Hampel, Clifford A. 1968. ''The Encyclopedia of the Chemical Elements.'' New York: Reinhold Book Corp. ISBN 0442155980. |
| − | * Morss, Lester R., Norman M. Edelstein, and Jean Fuger, eds. 2006. ''The Chemistry of the Actinide and Transactinide Elements'' | + | * Morss, Lester R., Norman M. Edelstein, and Jean Fuger, eds. 2006. ''The Chemistry of the Actinide and Transactinide Elements,'' 3rd ed. 5 vols. Joseph J. Katz, adapter. Dordrecht: Springer. ISBN 1402035551. |
| − | * Stwertka, Albert. 1998. ''Guide to the Elements'' | + | * Stwertka, Albert. 1998. ''Guide to the Elements,'' Rev. ed. Oxford: Oxford University Press. ISBN 0195080831. |
== External links == | == External links == | ||
| + | All links retrieved May 3, 2023. | ||
| + | * [http://www.eia.doe.gov/fuelnuclear.html Nuclear.] ''U.S. Energy Information Administration''. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
{{Nuclear Technology}} | {{Nuclear Technology}} | ||
| Line 320: | Line 270: | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
[[Category:Chemical elements]] | [[Category:Chemical elements]] | ||
| − | + | ||
{{credit|115047988}} | {{credit|115047988}} | ||
Latest revision as of 15:33, 16 January 2024
| |||||||||||||||||||||||||||||||||||||||||||
| General | |||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | uranium, U, 92 | ||||||||||||||||||||||||||||||||||||||||||
| Chemical series | actinides | ||||||||||||||||||||||||||||||||||||||||||
| Group, Period, Block | n/a, 7, f | ||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery gray metallic; corrodes to a spalling black oxide coat in air 
| ||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 238.02891(3) g/mol | ||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [Rn] 5f3 6d1 7s2 | ||||||||||||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 32, 21, 9, 2 | ||||||||||||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||||||||||||
| Phase | solid | ||||||||||||||||||||||||||||||||||||||||||
| Density (near r.t.) | 19.1 g/cm³ | ||||||||||||||||||||||||||||||||||||||||||
| Liquid density at m.p. | 17.3 g/cm³ | ||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1405.3 K (1132.2 °C, 2070 °F) | ||||||||||||||||||||||||||||||||||||||||||
| Boiling point | 4404 K (4131 °C, 7468 °F) | ||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 9.14 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| Heat of vaporization | 417.1 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| Heat capacity | (25 °C) 27.665 J/(mol·K) | ||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | orthorhombic | ||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3+,4+,5+,6+[1] (weakly basic oxide) | ||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.38 (Pauling scale) | ||||||||||||||||||||||||||||||||||||||||||
| Ionization energies | 1st: 597.6 kJ/mol | ||||||||||||||||||||||||||||||||||||||||||
| 2nd: 1420 kJ/mol | |||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 175 pm | ||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 186 pm | ||||||||||||||||||||||||||||||||||||||||||
| Miscellaneous | |||||||||||||||||||||||||||||||||||||||||||
| Magnetic ordering | paramagnetic | ||||||||||||||||||||||||||||||||||||||||||
| Electrical resistivity | (0 °C) 0.280 µΩ·m | ||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | (300 K) 27.5 W/(m·K) | ||||||||||||||||||||||||||||||||||||||||||
| Thermal expansion | (25 °C) 13.9 µm/(m·K) | ||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (20 °C) 3155 m/s | ||||||||||||||||||||||||||||||||||||||||||
| Speed of sound (thin rod) | (r.t.) 208 m/s | ||||||||||||||||||||||||||||||||||||||||||
| Shear modulus | 111 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Bulk modulus | 100 GPa | ||||||||||||||||||||||||||||||||||||||||||
| Poisson ratio | 0.23 | ||||||||||||||||||||||||||||||||||||||||||
| CAS registry number | 7440-61-1 | ||||||||||||||||||||||||||||||||||||||||||
| Notable isotopes | |||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||
Uranium (chemical symbol U, atomic number 92) is a silvery metallic chemical element in the actinide series of the periodic table. The heaviest naturally occurring element, uranium is nearly twice as dense as lead and weakly radioactive. It occurs naturally in low concentrations (a few parts per million) in soil, rock and water, and is commercially extracted from uranium-bearing minerals such as uraninite (see uranium mining).
In nature, uranium atoms exist as uranium-238 (99.275 percent), uranium-235 (0.72 percent), and a very small amount of uranium-234 (0.0058 percent). Uranium decays slowly by emitting an alpha particle. The half-life of uranium-238 is about 4.5 billion years and that of uranium-235 is 700 million years, making them useful in dating the age of the Earth. Along with thorium and plutonium, it is one of the three fissile elements, meaning it can easily break apart to become lighter elements. This property of uranium-235 and to a lesser degree uranium-233 generates the heat needed to run nuclear reactors and provides the explosive material for nuclear weapons. Both uses rely on the ability of uranium to produce a sustained nuclear chain reaction. Depleted uranium (uranium-238) is used in kinetic energy penetrators and armor plating.[2]
In addition to its uses in nuclear technology, uranium has been used as a colorant in uranium glass, producing orange-red through lemon yellow hues. It was also used for tinting in early photography.
Occurrence
Biotic and abiotic

Uranium is a naturally occurring element that can be found in low levels within all rock, soil, and water. Uranium is also the highest-numbered element to be found naturally in significant quantities on earth and is always found combined with other elements.[3] It, along with all elements with atomic weights higher than iron, are only naturally formed in supernova explosions.[4] The decay of uranium, thorium and potassium-40 in the Earth's mantle is thought to be the main source of heat[5][6] that keeps the outer core liquid and drives mantle convection, which in turn drives plate tectonics.
Its average concentration in the Earth's crust is (depending on the reference) 2 to 4 parts per million,[7][8] or about 40 times as abundant as silver.[9] The Earth's crust from the surface to 25 km (15 miles) down is calculated to contain 1017 kg (2 x 1017 lb) of uranium while the oceans may contain 1013 kg (2 x 1013 lb).[7] The concentration of uranium in soil ranges from 0.7 to 11 parts per million (up to 15 parts per million in farmland soil due to use of phosphate fertilizers) and 3 parts per billion of sea water is composed of the element.[8]
It is more plentiful than antimony, tin, cadmium, mercury, or silver and is about as abundant as arsenic or molybdenum.[8] It is found in hundreds of minerals including uraninite (the most common uranium ore), autunite, uranophane, torbernite, and coffinite. Significant concentrations of uranium occur in some substances such as phosphate rock deposits, and minerals such as lignite, and monazite sands in uranium-rich ores[3] (it is recovered commercially from these sources with as little as 0.1 percent uranium[9]).
It has been shown in some recent work at Manchester that bacteria can reduce and fix uranium in soils. This research is continuing at the university of Plymouth by Dr Keith Roach and S Handley. Some micro-organisms, such as the lichen Trapelia involuta or the bacterium Citrobacter, can absorb concentrations of uranium that are up to 300 times higher than their environment.[10] Citrobactor species absorb uranyl ions when given glycerol phosphate (or other similar organic phosphates). After one day, one gram of bacteria will encrust themselves with nine grams of uranyl phosphate crystals; creating the possibility that these organisms could be used to decontaminate uranium-polluted water.[11][12]
Plants absorb some uranium from the soil they are rooted in. Dry weight concentrations of uranium in plants range from 5 to 60 parts per billion and ash from burnt wood can have concentrations up to 4 parts per million.[11] Dry weight concentrations of uranium in food plants are typically lower with one to two micrograms per day ingested through the food people eat.[11]
Production and reserves
Uranium ore is mined in several ways: by open pit, underground or by leaching uranium from low-grade ores (see uranium mining).[2] Uranium ore typically contains 0.1 to 0.25 percent of actual uranium oxides so extensive measures must be employed to extract the metal from its ore.[13] Uranium ore is crushed and rendered into a fine powder and then leached with either an acid or alkali. The leachate is then subjected to one of several sequences of precipitation, solvent extraction, and ion exchange. The resulting mixture, called yellowcake, contains at least 75 percent uranium oxides. Yellowcake is then generally further refined using nitric acid to create a solution of uranyl nitrate. Additional solvent extraction procedures finish the process.[13]
Commercial-grade uranium can be produced through the reduction of uranium halides with alkali or alkaline earth metals. Uranium metal can also be made through electrolysis of KUF5 or UF4, dissolved in a molten calcium chloride (CaCl2) and sodium chloride (NaCl). Very pure uranium can be produced through the thermal decomposition of uranium halides on a hot filament.[3]
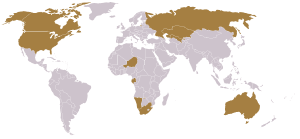
In 2005 seventeen countries produced concentrated uranium oxides; with Canada (27.9 percent) and Australia (22.8 percent) being the largest producers and Kazakhstan (10.5 percent), Russia (8.0 percent), Namibia (7.5 percent), Niger (7.4 percent), Uzbekistan (5.5 percent), the United States (2.5 percent), the Ukraine (1.9 percent), and China (1.7 percent) also producing significant amounts.[14] Three million metric ton of uranium ore reserves are known to exist and an additional five billion metric ton of uranium are estimated to be in sea water (Japanese scientists in the 1980s proved that extraction of uranium from sea water using ion exchangeers was feasible).[2]
Australia has the world's largest uranium ore reserves—40 percent of the planet's known supply. In fact, the world's largest single uranium deposit is located at the Olympic Dam Mine in South Australia.[15] Almost all the uranium is exported, but under strict International Atomic Energy Agency safeguards to satisfy the Australian people and government that none of the uranium is used in nuclear weapons. As of 2006, the Australian government was advocating an expansion of uranium mining, although issues with state governments and indigenous interests complicate the issue.[16]
The largest single domestic source of uranium in the United states was the Colorado Plateau located in Colorado, Utah, New Mexico, and Arizona. United States Federal government paid discovery bonuses and guaranteed purchase prices to anyone who found and delivered Uranium ore. The United States Government was the sole legal purchaser of the uranium. The economic incentives resulted in a frenzy of exploration and mining activity throughout the Colorado plateau from 1947 through 1959 that left thousands of miles of crudely graded roads spiderwebbing the remote deserts of the Colorado Plateau, and thousands of abandoned uranium mines, exploratory shafts, and tailings piles. The frenzy ended as suddenly as it had begun, when the U.S. governments stopped purchasing the uranium.
History
Pre-discovery use
The use of uranium, in its natural oxide form, dates back to at least 79 C.E., when it was used to add a yellow color to ceramic glazes.[3] Yellow glass with 1 percent uranium oxide was found in a Roman villa on Cape Posilipo in the Bay of Naples, Italy by R. T. Gunther of the University of Oxford in 1912.[17] Starting in the late Middle Ages, pitchblende was extracted from the Habsburg silver mines in Joachimsthal, Bohemia (now in the Czech Republic) and was used as a coloring agent in the local glassmaking industry.[11] In the early nineteenth century, the world's only known source of uranium ores were these old mines.
Discovery
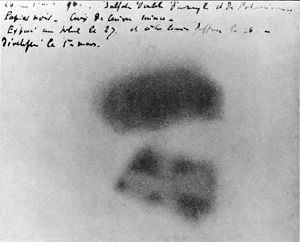
The discovery of the element is credited to the German pharmacist Martin Heinrich Klaproth, who named the new element after the planet Uranus. While working in his experimental laboratory in Berlin in 1789, Klaproth was able to precipitate a yellow compound (likely sodium diuranate) by dissolving pitchblende in nitric acid and neutralizing the solution with sodium hydroxide.[11] Klaproth mistakenly assumed the yellow substance was the oxide of a yet-undiscovered element and heated it with charcoal to obtain a black powder, which he thought was the newly discovered metal itself (in fact, that powder was an oxide of uranium).[11][18] He named the newly discovered element after the planet Uranus, which had been discovered eight years earlier by William Herschel. In 1841, Eugene-Melchior Peligot, who was Professor of Analytical Chemistry at the Central School of Arts and Manufactures in Paris, isolated the first sample of uranium metal by heating uranium tetrachloride with potassium.[19][11] Uranium was not seen as being particularly dangerous during much of the nineteenth century, leading to the development of various uses for the element. One such use for the oxide was the coloring of pottery and glass.
Antoine Becquerel discovered radioactivity by using uranium in 1896.[9] Becquerel made the discovery in Paris by leaving a sample of uranium on top of an unexposed photographic plate in a drawer and noting that the plate had become 'fogged'.[20] He determined that a form of invisible light or rays emitted by uranium had exposed the plate.
Fission research

A team led by Enrico Fermi in 1934 observed that bombarding uranium with neutrons produces the emission of beta rays (electrons or positrons; see beta particle).[21] The experiments leading to the discovery of uranium's ability to fission (break apart) into lighter elements and release binding energy were conducted by Otto Hahn and Fritz Strassmann[21] in Hahn's laboratory in Berlin. Lise Meitner and her nephew, physicist Otto Robert Frisch, published the physical explanation in February 1939 and named the process 'nuclear fission'.[22] Soon after, Fermi hypothesized that the fission of uranium might release enough neutrons to sustain a fission reaction. Confirmation of this hypothesis came in 1939 and later work found that 2 1/2 neutrons are released by each fission of the rare uranium isotope uranium-235.[21] Further work found that the far more common uranium-238 isotope can be transmuted into plutonium, which, like uranium-235, is also fissionable by thermal neutrons.
On December 2, 1942, another team led by Enrico Fermi was able to initiate the first artificial nuclear chain reaction. Working in a lab below the stands of Stagg Field at the University of Chicago, the team created the conditions needed for such a reaction by piling together 400 tons (360 metric tons) of graphite, 58 tons (53 metric tons) of uranium oxide, and six tons (five and a half metric tons) of uranium metal.[21] Later researchers found that such a chain reaction could either be controlled to produce usable energy or could be allowed to go out of control to produce an explosion more violent than anything possible using chemical explosives.
Bombs and reactors

Two major types of atomic bomb were developed in the Manhattan Project during World War II: a plutonium-based device (see Trinity test and 'Fat Man') whose plutonium was derived from uranium-238, and a uranium-based device (nicknamed 'Little Boy') whose fissile material was highly enriched uranium. The uranium-based Little Boy device became the first nuclear weapon used in war when it was detonated over the Japanese city of Hiroshima on August 6, 1945. Exploding with a yield equivalent to 12,500 metric tons of TNT, the blast and thermal wave of the bomb destroyed nearly 50,000 buildings and killed approximately 75,000 people (see Atomic bombings of Hiroshima and Nagasaki).[20] Initially it was believed that uranium was relatively rare, and that nuclear proliferation could be avoided by simply buying up all known uranium stocks, but within a decade large deposits of it were discovered in many places around the world.

Experimental Breeder Reactor I at the Idaho National Engineering and Environmental Laboratory near Arco, Idaho became the first functioning artificial nuclear reactor on December 20 1951. Initially, only four 150-watt light bulbs were lit by the reactor but improvements eventually enabled it to power the whole facility (later, the whole town of Arco became the first in the world to have all its electricity come from nuclear power).[23] The world's first commercial scale nuclear power station, Calder Hall, in England, began generation on October 17 1956.[24] Another early power reactor was the Shippingport Reactor in Pennsylvania, which began electricity production in 1957. Nuclear power was used for the first time for propulsion by a submarine, the USS Nautilus, in 1954.[21]
Fifteen ancient and no longer active natural fission reactors were found in three separate ore deposits at the Oklo mine in Gabon, West Africa in 1972. Discovered by French physicist Francis Perrin, they are collectively known as the Oklo Fossil Reactors. The ore they exist in is 1.7 billion years old; at that time, uranium-235 comprised about three percent of the total uranium on Earth.[25] This is high enough to permit nuclear fission to occur, providing other conditions are right. The ability of the surrounding sediment to contain the nuclear waste products in less than ideal conditions has been cited by the U.S. federal government as evidence of their claim that the Yucca Mountain facility could safely be a repository of waste for the nuclear power industry.[25]
Cold War legacy and waste
During the Cold War between the Soviet Union and the United States, huge stockpiles of uranium were amassed and tens of thousands of nuclear weapons were created, using enriched uranium and plutonium made from uranium.
Since the break-up of the Soviet Union in 1991, an estimated 600 tons (540 metric tons) of highly-enriched weapons grade uranium (enough to make 40,000 nuclear warheads) have been stored in often inadequately guarded facilities in the Russian Federation and several other former Soviet states.[26] Police in Asia, Europe, and South America on at least 16 occasions from 1993 to 2005 have intercepted shipments of smuggled bomb-grade uranium or plutonium, most of which was from ex-Soviet sources.[26] From 1993 to 2005 the Material Protection, Control, and Accounting Program, operated by the federal government of the United States, spent approximately US$550 million to help safeguard uranium and plutonium stockpiles in Russia.[26]
Nuclear fallout and pollution have occurred from above-ground nuclear tests[27] and several nuclear accidents: the Windscale fire at the Sellafield nuclear plant in 1957 spread iodine-131 over much of Northern England, the Three Mile Island accident in 1979 released radon gas and some iodine-131, the Chernobyl disaster in 1986 released radon, iodine-131 and strontium-90 that spread over much of Europe.[8]
Notable characteristics
Uranium is an inner transition metal of the actinide series, situated in period 7 of the periodic table, between protactinium and neptunium. When refined, it is a silvery white, weakly radioactive metal, which is slightly softer than steel,[3] strongly electropositive and a poor electrical conductor.[7] It is malleable, ductile, and slightly paramagnetic.[3] Uranium metal has very high density, 65 percent more dense than lead, but slightly less dense than gold.
Uranium metal reacts with nearly all nonmetallic elements and their compounds with reactivity increasing with temperature.[9] Hydrochloric and nitric acids dissolve uranium but nonoxidizing acids attack the element very slowly.[7] When finely divided, it can react with cold water; in air, uranium metal becomes coated with a dark layer of uranium oxide.[3] Uranium in ores is extracted chemically and converted into uranium dioxide or other chemical forms usable in industry.
Uranium was the first element that was found to be fissile. Upon bombardment with slow neutrons, its uranium-235 isotope becomes a very short lived uranium-236 isomer which immediately divides into two smaller nuclei, releasing nuclear binding energy and more neutrons. If these neutrons are absorbed by other uranium-235 nuclei, a nuclear chain reaction occurs and, if there is nothing to absorb some neutrons and slow the reaction, the reaction is explosive. As little as 15 lb (7 kg) of uranium-235 can be used to make an atomic bomb.[26] The first atomic bomb worked by this principle (nuclear fission).
Uranium metal has three allotropic forms:
- alpha (orthorhombic) stable up to 667.7 °C
- beta (tetragonal) stable from 667.7 °C to 774.8 °C
- gamma (body-centered cubic) from 774.8 °C to melting point - this is the most malleable and ductile state.
Isotopes
Natural concentrations
Naturally occurring uranium is composed of three major isotopes, uranium-238 (99.28 percent natural abundance), uranium-235 (0.71 percent), and uranium-234 (0.0054 percent). All three isotopes are radioactive, creating radioisotopes, with the most abundant and stable being uranium-238 with a half-life of 4.51 × 109 years (close to the age of the Earth), uranium-235 with a half-life of 7.13 × 108 years, and uranium-234 with a half-life of 2.48 × 105 years.[28]
Uranium-238 is an α emitter, decaying through the 18-member uranium natural decay series into lead-206.[9] The decay series of uranium-235 (also called actinouranium) has 15 members that ends in lead-207, protactinium-231 and actinium-227.[9] The constant rates of decay in these series makes comparison of the ratios of parent to daughter elements useful in radiometric dating. Uranium-233 is made from thorium-232 by neutron bombardment.[3]
The isotope uranium-235 or enriched uranium is important for both nuclear reactors and nuclear weapons because it is the only isotope existing in nature to any appreciable extent that is fissile, that is, can be broken apart by thermal neutrons.[9] The isotope uranium-238 is also important because it absorbs neutrons to produce a radioactive isotope that subsequently decays to the isotope plutonium-239, which also is fissile.[21]
Enrichment
Enrichment of uranium ore through isotope separation to concentrate the fissionable uranium-235 is needed for use in nuclear power plants and nuclear weapons. A majority of neutrons released by a fissioning atom of uranium-235 must impact other uranium-235 atoms to sustain the nuclear chain reaction needed for these applications. The concentration and amount of uranium-235 needed to achieve this is called a 'critical mass.'
To be considered 'enriched' the uranium-235 fraction has to be increased to significantly greater than its concentration in naturally-occurring uranium. Enriched uranium typically has a uranium-235 concentration of between 3 and 5 percent.[29] The process produces huge quantities of uranium that is depleted of uranium-235 and with a correspondingly increased fraction of uranium-238, called depleted uranium or 'DU'. To be considered 'depleted', the uranium-235 isotope concentration has to have been decreased to significantly less than its natural concentration.
The gas centrifuge process, where gaseous uranium hexafluoride (UF6) is separated by weight using high-speed centrifuges, has become the cheapest and leading enrichment process (lighter UF6 concentrates in the center of the centrifuge).[20] The gaseous diffusion process was the previous leading method for enrichment and the one used in the Manhattan Project. In this process, uranium hexafluoride is repeatedly diffused through a silver-zinc membrane and the different isotopes of uranium are separated by diffusion rate (uranium 238 is heavier and thus diffuses slightly slower than uranium-235).[20] The laser excitation method employs a laser beam of precise energy to sever the bond between uranium-235 and fluorine. This leaves uranium-238 bonded to fluorine and allows uranium-235 metal to precipitate from the solution.[2] Another method is called liquid thermal diffusion.[7]
Compounds
Oxidation states/Oxides
Ions that represent the four different oxidation states of uranium are soluble and therefore can be studied in aqueous solutions. They are: U3+ (red), U4+ (green), UO2+ (unstable), and UO2+ (yellow).[30] A few solid and semi-metallic compounds such as UO and US exist for the formal oxidation state uranium(II) but no simple ions are known to exist in solution for that state. Ions of U3+liberate hydrogen from water and are therefore considered to be highly unstable. The UO2+ ion represents the uranium(V) state and is known to form compounds that include inorganic ions such as carbonate, chloride and sulfate, and various organic chelating agents.[30]
Phase relationships in the uranium-oxygen system are highly complex. The most important oxidation states of uranium are uranium(IV) and uranium(VI) and their two corresponding oxides are, respectively, uranium dioxide (UO2) and uranium trioxide (UO3).[31] Other uranium oxides, such as uranium monoxide (UO), diuranium pentoxide (U2O5), and uranium peroxide (UO4•2H2O) are also known to exist.
The most common forms of uranium oxide are triuranium octaoxide (U3O8) and the aforementioned UO2.[32] Both oxide forms are solids that have low solubility in water and are relatively stable over a wide range of environmental conditions. Triuranium octaoxide is (depending on conditions) the most stable compound of uranium and is the form most commonly found in nature. Uranium dioxide is the form in which uranium is most commonly used as a nuclear reactor fuel.[32] At ambient temperatures, UO2 will gradually convert to U3O8. Because of their stability, uranium oxides are generally considered the preferred chemical form for storage or disposal.[32]
Hydrides, carbides and nitrides
Uranium metal heated to 250 to 300 °C reacts with hydrogen to form uranium hydride. Yet higher temperatures will reversibly remove the hydrogen. This property makes uranium hydrides convenient starting materials to create reactive uranium powder along with various uranium carbide, nitride, and halide compounds.[33] Two crystal modifications of uranium hydride exist: an α form that is obtained at low temperatures and a β form that is created when the formation temperature is above 250 °C.[33]
Uranium carbides and uranium nitrides are both relatively inert semimetallic compounds that are minimally soluble in acids, react with water, and can ignite in air to form U3O8.[33] Carbides of uranium include uranium monocarbide (UC), uranium dicarbide (UC2), and diuranium tricarbide (U2C3). Both UC and UC2 are formed by adding carbon to molten uranium or by exposing the metal to carbon monoxide at high temperatures. Stable below 1800 °C, U2C3 is prepared by subjecting a heated mixture of UC and UC2 to mechanical stress.[34] Uranium nitrides obtained by direct exposure of the metal to nitrogen include uranium mononitride (UN), uranium dinitride (UN2), and diuranium trinitride (U2N3).[34]
Halides
All uranium fluorides are created using uranium tetrafluoride (UF4); UF4 itself is prepared by hydrofluorination or uranium dioxide.[33] Reduction of UF4 with hydrogen at 1000 °C produces uranium trifluoride (UF3). Under the right conditions of temperature and pressure, the reaction of solid UF4 with gaseous uranium hexafluoride (UF6) can form the intermediate fluorides of U2F9, U4F17, and UF5.[33]
At room temperatures, UF6 has a high vapor pressure, making it useful in the gaseous diffusion process to separate highly valuable uranium-235 from the far more common uranium-238 isotope. This compound can be prepared from uranium dioxide and uranium hydride by the following process:[33]
UO2 + 4HF + heat (500 °C) → UF4 + 2H2O
UF4 + F2 + heat (350°) → UF6
The resulting UF6 white solid is highly reactive (by fluorination), easily sublimes (emitting a nearly perfect gas vapor), and is the most volatile compound of uranium known to exist.[33]
One method of preparing uranium tetrachloride (UCl4) is to directly combine chlorine with either uranium metal or uranium hydride. The reduction of UCl4 by hydrogen produces uranium trichloride (UCl3) while the higher chlorides of uranium are prepared by reaction with additional chlorine.[33] All uranium chlorides react with water and air.
Bromides and iodides of uranium are formed by direct reaction of, respectively, bromine and iodine with uranium or by adding UH3 to those element's acids.[33] Known examples include: UBr3, UBr4, UI3, and UI4. Uranium oxyhalides are water-soluble and include UO2F2, UOCl2, UO2Cl2, and UO2Br2. Stability of the oxyhalides decrease as the atomic weight of the component halide increases.[33]
Applications
Military
The major application of uranium in the military sector is in high-density penetrators. This ammunition consists of depleted uranium (DU) alloyed with 1–2% other elements. At high impact speed, the density, hardness, and flammability of the projectile enable destruction of heavily armored targets. Tank armor and the removable armor on combat vehicles are also hardened with depleted uranium (DU) plates. The use of DU became a contentious political-environmental issue after U.S., UK and other countries' use of DU munitions in wars in the Persian Gulf and the Balkans raised questions of uranium compounds left in the soil (see Gulf War Syndrome).[26]
Depleted uranium is also used as a shielding material in some containers used to store and transport radioactive materials.[7] Other uses of DU include counterweights for aircraft control surfaces, as ballast for missile re-entry vehicles and as a shielding material.[3] Due to its high density, this material is found in inertial guidance devices and in gyroscopic compasses.[3] DU is preferred over similarly dense metals due to its ability to be easily machined and cast.[8]
During the later stages of World War II, the entire Cold War and to a much lesser extent afterwards, uranium was used as the fissile explosive material to produce nuclear weapons. Two major types of fission bombs were built: a relatively simple device that uses uranium-235 and a more complicated mechanism that uses uranium-238-derived plutonium-239. Later, a much more complicated and far more powerful fusion bomb that uses a plutonium-based device in a uranium casing to cause a mixture of tritium and deuterium to undergo nuclear fusion was built.[35]
Civilian
The main use of uranium in the civilian sector is to fuel commercial nuclear power plants; by the time it is completely fissioned, one kilogram of uranium can theoretically produce about 20 trillion joules of energy (20 × 1012 joules); as much electricity as 1500 metric ton of coal.[2] Generally this is in the form of enriched uranium, which has been processed to have higher-than-natural levels of uranium-235 and can be used for a variety of purposes relating to nuclear fission.
Commercial nuclear power plants use fuel that is typically enriched to around 3% uranium-235,[2] though some reactor designs (such as the CANDU reactors) can use unenriched uranium fuel. Fuel used for United States Navy submarine reactors is typically highly enriched in uranium-235 (the exact values are classified). In a breeder reactor, uranium-238 can also be converted into plutonium through the following reaction:[3] 238U(n, gamma) -> 239U -(beta)-> 239Np -(beta)-> 239Pu.
Prior to the discovery of radiation, uranium was primarily used in small amounts for yellow glass and pottery dyes (such as uranium glass and in Fiestaware). Uranium was also used in photographic chemicals (esp. uranium nitrate as a toner),[3] in lamp filaments, to improve the appearance of dentures, and in the leather and wood industries for stains and dyes. Uranium salts are mordants of silk or wool. The discovery of radiation in uranium ushered-in additional scientific and practical uses of the element.
The long half-life of the isotope uranium-238 (4.51 × 109 years) make it well-suited for use in estimating the age of the earliest igneous rocks and for other types of radiometric dating (including uranium-thorium dating and uranium-lead dating). Uranium metal is used for X-ray targets in the making of high-energy X-rays.[3]
Precautions
Exposure
A person can be exposed to uranium (or its radioactive daughters such as radon) by inhaling dust in air or by ingesting contaminated water and food. The amount of uranium in air is usually very small; however, people who work in factories that process phosphate fertilizers, live near government facilities that made or tested nuclear weapons, or live or work near a coal-fired power plant, facilities that mine or process uranium ore, or enrich uranium for reactor fuel, may have increased exposure to uranium.[36][37] Houses or structures which are over uranium deposits (either natural or man-made slag deposits) may have an increased incidence of exposure to radon gas.
Almost all uranium that is ingested is excreted during digestion, but up to 5 percent is absorbed by the body when the soluble uranyl ion is ingested while only 0.5 percent is absorbed when insoluble forms of uranium, such as its oxide, are ingested.[11] However, soluble uranium compounds tend to quickly pass through the body whereas insoluble uranium compounds, especially when ingested via dust into the lungs, pose a more serious exposure hazard. After entering the bloodstream, the absorbed uranium tends to bioaccumulate and stay for many years in bone tissue because of uranium's affinity for phosphates.[11] Uranium does not absorb through the skin, and alpha particles released by uranium cannot penetrate the skin.
Effects
The greatest health risk from large intakes of uranium is toxic damage to the kidneys, because, in addition to being weakly radioactive, uranium is a toxic metal.[38][11] Radiological effects are generally local because this is the nature of alpha radiation, the primary form from U-238 decay. No human cancer of any type has ever been seen as a result of exposure to natural or depleted uranium[39] but exposure to some of its decay products, especially radon, strontium-90, and iodine-131 does pose a significant health threat.[8]
Although accidental inhalation exposure to a high concentration of uranium hexafluoride has resulted in human fatalities, those deaths were not associated with uranium itself.[40] Finely-divided uranium metal presents a fire hazard because uranium is pyrophoric, so small grains will ignite spontaneously in air at room temperature.[3]
See also
- Chemical element
- Inner transition metal
- Metal
- Nuclear engineering
- Nuclear physics
- Periodic table
- Radioactive decay
Notes
- ↑ L.R. Morss, N.M. Edelstein, J. Fuger, (eds.) The Chemistry of the Actinide and Transactinide Elements, Third Edition. (Netherlands: Springer, 2006.)
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 John Emsley. Nature's Building Blocks. (Oxford: Oxford Univ. Press, 2001), 479
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 Uranium. Los Alamos National Laboratory accessdate 2007-01-14
- ↑ WorldBook@NASA: Supernova NASA. accessdate 2007-02-19
- ↑ Celeste Biever, First measurements of Earth's core radioactivity New Scientist 27 July 2005
- ↑ Potassium-40 heats up Earth's core physicsweb.org May 7, 2003. accessdate 2007-01-14
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Uranium." The McGraw-Hill Science and Technology Encyclopedia, 5th ed. (The McGraw-Hill Companies, Inc.) [1]. Retrieved October 30, 2008.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Emsley, 480
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 "uranium." Columbia Electronic Encyclopedia, 6th Ed. Columbia University Press
- ↑ Emsley, 476, 482
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 11.6 11.7 11.8 11.9 Emsley, 477
- ↑ L. E. Macaskie, R. M. Empson, A. K. Cheetham, C. P. Grey, A. J. Skarnulis, Uranium bioaccumulation by a Citrobacter sp. as a result of enzymically mediated growth of polycrystalline HUO2PO4. Science 257 (1992): 782-784. doi = 10.1126/science.1496397
- ↑ 13.0 13.1 Seaborg, Encyclopedia of the Chemical Elements (1968), 774
- ↑ World Uranium Production. UxC Consulting Company, LLC
- ↑ Uranium Mining and Processing in South Australia South Australian Chamber of Mines and Energy 2002. accessdate 2007-01-14
- ↑ "Nuclear Balance of Power," BRW, 26 Oct. 2006, 41–44
- ↑ Emsley, 482
- ↑ M. H. Klaproth, Chemische Untersuchung des Uranits, einer neuentdeckten metallischen Substanz. Chemische Annalen 2 (1789): 387-403
- ↑ E.-M. Peligot, Recherches Sur L'Uranium. Annales de chimie et de physique 5 (5) (1842): 5-47 [2]
- ↑ 20.0 20.1 20.2 20.3 Emsley, 478
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 Seaborg, Encyclopedia of the Chemical Elements (1968), 773
- ↑ L. Meitner, O. Frisch. Disintegration of Uranium by Neutrons: a New Type of Nuclear Reaction. Nature 143 (1939): 239-240. doi 10.1038/224466a0. [3]
- ↑ History and Success of Argonne National Laboratory: Part 1. U.S. Department of Energy, Argonne National Laboratory 1998. accessdate 2007-01-28
- ↑ BBC News, On this Day 1956: Queen switches on nuclear power accessdate June 28, 2006
- ↑ 25.0 25.1 Oklo: Natural Nuclear Reactors Office of Civilian Radioactive Waste Management accessdate June 28 2006
- ↑ 26.0 26.1 26.2 26.3 26.4 Encyclopedia of Espionage, Intelligence, and Security. The Gale Group, Inc. uranium [4]
- ↑ T. Warneke, I. W. Croudace, P. E. Warwick, R. N. Taylor. A new ground-level fallout record of uranium and plutonium isotopes for northern temperate latitudes. Earth and Planetary Science Letters 2002 203 (3-4):1047-1057. doi = 10.1016/S0012-821X(02)00930-5
- ↑ Seaborg, 777
- ↑ Uranium Enrichment Argonne National Laboratory. accessdate 2007-02-11
- ↑ 30.0 30.1 Seaborg, Encyclopedia of the Chemical Elements (1968), 778
- ↑ Seaborg, 779
- ↑ 32.0 32.1 32.2 Chemical Forms of Uranium Argonne National Laboratory. accessdate 2007-02-18
- ↑ 33.0 33.1 33.2 33.3 33.4 33.5 33.6 33.7 33.8 33.9 Seaborg, 782
- ↑ 34.0 34.1 Seaborg, 780
- ↑ Nuclear Weapon Design Federation of American Scientists 1998. accessdate 2007-02-19
- ↑ Radiation Information for Uranium U.S. Environmental Protection Agency accessdate 2007-02-18
- ↑ ToxFAQ for Uranium Agency for Toxic Substances and Disease Registry September 1999. accessdate 2007-02-18
- ↑ Toxicological Profile for Uranium [5] Agency for Toxic Substances and Disease Registry (ATSDR) Atlanta, GA. CAS# 7440-61-1 September 1999
- ↑ Public Health Statement for Uranium CDC accessdate 2007-02-15
- ↑ Kathren and Moore 1986; Moore and Kathren 1985; USNRC 1986
ReferencesISBN links support NWE through referral fees
- Emsley, John. 2001. Nature's Building Blocks: An A–Z Guide to the Elements. Oxford: Oxford Univ. Press. ISBN 0198503407.
- Greenwood, N.N., and A. Earnshaw. 1998. Chemistry of the Elements, 2nd ed. Oxford, UK; Burlington, MA: Butterworth-Heinemann. ISBN 0750633654. Online version.
- Hampel, Clifford A. 1968. The Encyclopedia of the Chemical Elements. New York: Reinhold Book Corp. ISBN 0442155980.
- Morss, Lester R., Norman M. Edelstein, and Jean Fuger, eds. 2006. The Chemistry of the Actinide and Transactinide Elements, 3rd ed. 5 vols. Joseph J. Katz, adapter. Dordrecht: Springer. ISBN 1402035551.
- Stwertka, Albert. 1998. Guide to the Elements, Rev. ed. Oxford: Oxford University Press. ISBN 0195080831.
External links
All links retrieved May 3, 2023.
- Nuclear. U.S. Energy Information Administration.
| Nuclear technology | |
|---|---|
| Nuclear engineering | Nuclear physics | Nuclear fission | Nuclear fusion | Radiation | Ionizing radiation | Atomic nucleus | Nuclear reactor | Nuclear safety |
| Nuclear material | Nuclear fuel | Fertile material | Thorium | Uranium | Enriched uranium | Depleted uranium | Plutonium |
| Nuclear power | Nuclear power plant | Radioactive waste | Fusion power | Future energy development | Inertial fusion power plant | Pressurized water reactor | Boiling water reactor | Generation IV reactor | Fast breeder reactor | Fast neutron reactor | Magnox reactor | Advanced gas-cooled reactor | Gas-cooled fast reactor | Molten salt reactor | Liquid-metal-cooled reactor | Lead-cooled fast reactor | Sodium-cooled fast reactor | Supercritical water reactor | Very high temperature reactor | Pebble bed reactor | Integral Fast Reactor | Nuclear propulsion | Nuclear thermal rocket | Radioisotope thermoelectric generator |
| Nuclear medicine | PET | Radiation therapy | Tomotherapy | Proton therapy | Brachytherapy |
| Nuclear weapons | History of nuclear weapons | Nuclear warfare | Nuclear arms race | Nuclear weapon design | Effects of nuclear explosions | Nuclear testing | Nuclear delivery | Nuclear proliferation | List of states with nuclear weapons | List of nuclear tests |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.