Sublimation (chemistry)
In chemistry, sublimation is the process by which a substance undergoes conversion from the solid phase to the gas phase, without going through an intermediate liquid phase. Iodine crystals and solidified carbon dioxide are examples of substances that sublimate at room temperature and regular atmospheric pressure. By contrast, at normal pressures, most chemical compounds and elements possess three different states‚ÄĒsolid, liquid, and gas‚ÄĒat different temperatures. In these cases, the transition from the solid to the gaseous state requires an intermediate liquid state.[1]
The antonym (opposite process) of sublimation is called deposition. The formation of frost is an example of meteorological deposition.
Principles of sublimation
Sublimation is a phase transition that occurs at temperatures and pressures below what is called the "triple point" of the substance (see phase diagram). The process is an endothermic change‚ÄĒthat is, a change in which heat is absorbed by the system. The enthalpy of sublimation can be calculated as the enthalpy of fusion plus the enthalpy of vaporization.
Examples of sublimation
As mentioned above, carbon dioxide (CO2) is a common example of a chemical compound that sublimates at atmospheric pressure‚ÄĒa block of solid CO2 (dry ice) at room temperature and one atmosphere pressure will turn into gas without first becoming a liquid. Iodine is another substance that visibly sublimates at room temperature. In contrast to CO2, though, it is possible to obtain liquid iodine at atmospheric pressure by heating it.
Snow and other water ices also sublimate, although more slowly, at below-freezing temperatures. This phenomenon, used in freeze drying, allows a wet cloth to be hung outdoors in freezing weather and retrieved later in a dry state. Naphthalene, a common ingredient in mothballs, also sublimes slowly. Arsenic can also sublimate at high temperatures.
Some materials, such as zinc and cadmium, sublimate at low pressures. In high-vacuum applications, this phenomenon may be problematic.
Historical usage
In ancient alchemy, a protoscience that contributed to the development of modern chemistry and medicine, alchemists developed a structure of basic laboratory techniques, theory, terminology, and experimental methods. Sublimation was used to refer to the process in which a substance is heated to a vapor, then immediately collects as sediment on the upper portion and neck of the heating medium (typically a retort or alembic), but can also be used to describe other similar non-laboratory transitions.
It was mentioned by alchemical authors such as Basil Valentine and George Ripley, and in the Rosarium philosophorum, as a process necessary for the completion of the magnum opus. Here, the word sublimation was used to describe an exchange of "bodies" and "spirits" similar to laboratory phase transition between solids and gases. Valentine, in his Le char triomphal de l'antimoine (Triumphal Chariot of Antimony, published 1646)[2] made a comparison to spagyrics in which a vegetable sublimation can be used to separate the spirits in wine and beer.[3] Ripley used language more indicative of the mystical implications of sublimation, indicating that the process has a double aspect in the spiritualization of the body and the corporalizing of the spirit.[4]
- And Sublimations we make for three causes,
- The first cause is to make the body spiritual.
- The second is that the spirit may be corporeal,
- And become fixed with it and consubstantial.
- The third cause is that from its filthy original.
- It may be cleansed, and its saltiness sulphurious
- May be diminished in it, which is infectious.[5]
Contemporary uses
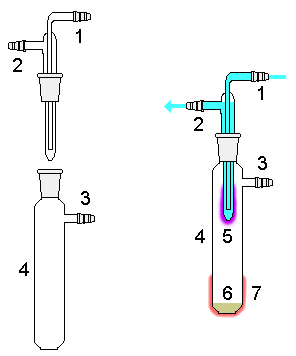
Sublimation is a technique used by chemists to purify compounds. Typically a solid is placed in a vessel which is then heated under vacuum. Under this reduced pressure the solid volatilizes and condenses as a purified compound on a cooled surface, leaving the non-volatile impurities behind. This cooled surface often takes the form of a "cold finger" (shown in the diagram above). Once heating ceases and the vacuum is released, the sublimed compound can be collected from the cooled surface. Usually this is done using a sublimation apparatus‚Äé.
Frost-free freezers are the result of having a fan and air circulation inside the freezer. The sub-zero temperature combined with the air circulation that keeps the air arid, significantly accelerates the sublimation process. This keeps freezer walls and shelves free of ice, although ice-cubes will continually sublimate.
Dye sublimation is also often used in color printing on a variety of substrates, including paper. A small heater is used to vaporize the solid dye material, which then solidifies upon the paper. As this type of printer allows extremely fine control of the primary color ratios it is possible to obtain a good quality picture even with relatively low printer resolution, as compared to other printer types of similar resolution. Standard black and white laser printers are capable of printing on plain paper using a special "transfer toner" containing sublimation dyes which can then be permanently heat transferred to T-shirts, hats, mugs, metals, puzzles and other surfaces.
In alchemy, sublimation typically refers to the process by which a substance is heated to a vapor, then immediately collects as sediment on the upper portion and neck of the heating medium (typically a retort or alembic). It is one of the 12 core alchemical processes.
In the Fast-Freeze, Deep-Etch technique, samples (for example, tissue samples) are rapidly frozen in liquid nitrogen and transferred to a vacuum device in which surface ice is sublimed. This effectively etches the sample surface, revealing the preserved 3D structure of the hydrated material. A rotary shadowed surface replica can then be obtained via electron microscopy.
Sublimation is also used to create freeze-dried substances, for example tea, soup or drugs in a process called lyophilization, which consists of freezing a solution or suspension and heating it very slowly under medium to high vacuum‚ÄĒspecifically, a pressure lower than the vapor pressure of the solvent at its melting point. This can be well below the melting point of water if there are organic solvents or salts in the sample being freeze-dried. The resulting solid is usually much easier to dissolve or resuspend than one that is produced from a liquid system, and the low temperatures involved cause less damage to sensitive or reactive substances.
Notes
- ‚ÜĎ Note that the pressure referred to here is the vapor pressure of the substance, not the total pressure of the entire system.
- ‚ÜĎ Basil Valentine, Triumphal Chariot of Antimony (Legare Street Press, 2022 (original 1646), ISBN 978-1015671607).
- ‚ÜĎ Francis Barrett, Lives of Alchemystical Philosophers (Lettel Books, 2024, (original 1815)).
- ‚ÜĎ Barbara Dibernard, Alchemy and Finnegans Wake (State University of New York Press, 1980, ISBN 978-0873953887).
- ‚ÜĎ George Ripley, The Compound of Ancient Alchemy (Kessinger Publishing, 2005 (original 1591), ISBN 978-1417916573).
ReferencesISBN links support NWE through referral fees
- Barrett, Francis. Lives of Alchemystical Philosophers. Lettel Books, 2024, (original 1815). ASIN B0CVW3HCY3
- Brady, James E., and Fred Senese. Chemistry: Matter and Its Changes. Wiley, 2004. ISBN 0471215171
- Clugston, Michael, and Rosalind Fleming. Advanced Chemistry. Oxford: Oxford University, 2000. ISBN 0199146330
- Dibernard, Barbara. Alchemy and Finnegans Wake. State University of New York Press, 1980. ISBN 978-0873953887
- Ripley, George. The Compound of Ancient Alchemy. Kessinger Publishing, 2005 (original 1591). ISBN 978-1417916573
- Smiley, Robert A., and Harold L. Jackson. Chemistry and the Chemical Industry: A Practical Guide for Non-Chemists. CRC Press, 2002. ISBN 1587160544
- Valentine, Basil. Triumphal Chariot of Antimony. Legare Street Press, 2022 (original 1646). ISBN 978-1015671607
External links
All links retrieved December 18, 2025.
- Sublimation Definition (Phase Transition in Chemistry) ThoughtCo.
- Sublimation and deposition Energy Education
- Sublimation ThoughtCo.
- Sublimation Chemistry Learner
- What is Sublimation Printing and How Does it Work? Your Questions Answered Printful
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.