Nuclear fission
| Nuclear physics | ||||||||||||||
| ||||||||||||||
| Radioactive decay Nuclear fission Nuclear fusion
| ||||||||||||||
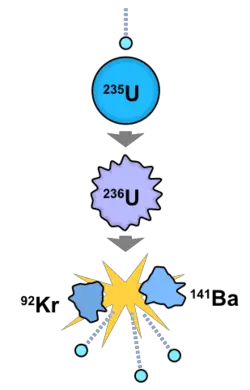
Nuclear fission is the splitting of the nucleus of an atom into parts (lighter nuclei), often producing photons (in the form of gamma rays), free neutrons, and other subatomic particles as by-products. Fission of heavy elements is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). Fission is a form of elemental transmutation because the resulting fragments are not the same element as the original atom.
Nuclear fission produces energy for nuclear power and to drive the explosion of nuclear weapons. Both uses are made possible because certain substances, called nuclear fuels, undergo fission when struck by free neutrons and in turn generate neutrons when they break apart. This makes possible a self-sustaining chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.
The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very attractive source of energy. However, the products of nuclear fission are radioactive and remain so for significantly long periods of time, leading to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.
Physical overview
Nuclear fission differs from other forms of radioactive decay in that it can be harnessed and controlled via a chain reaction: Free neutrons released by each fission event can trigger yet more events, which in turn release more neutrons and cause more fissions. Chemical isotopes that can sustain a fission chain reaction are called nuclear fuels, and are said to be fissile. The most common nuclear fuels are 235U (the isotope of uranium with an atomic mass of 235 and of use in nuclear reactors, 238) and 239Pu (the isotope of plutonium with an atomic mass of 239). These fuels break apart into a range of chemical elements with atomic masses near 100 (fission products). Most nuclear fuels undergo spontaneous fission only very slowly, decaying mainly via an alpha/beta decay chain over periods of millennia to eons. In a nuclear reactor or nuclear weapon, most fission events are induced by bombardment with another particle, such as a neutron.
Typical fission events release several hundred million eV of energy for each fission event. By contrast, most chemical oxidation reactions (such as burning coal or TNT) release at most a few eV per event, so nuclear fuel contains at least ten million times more usable energy than does chemical fuel. The energy of nuclear fission is released as kinetic energy of the fission products and fragments, and as electromagnetic radiation in the form of gamma rays; in a nuclear reactor, the energy is converted to heat as the particles and gamma rays collide with the atoms that make up the reactor and its working fluid, usually water or occasionally heavy water.
Nuclear fission of heavy elements produces energy because the specific binding energy (binding energy per mass) of intermediate-mass nuclei with atomic numbers and atomic masses close to 61Ni and 56Fe is greater than the specific binding energy of very heavy nuclei, so that energy is released when heavy nuclei are broken apart.
The total rest masses of the fission products (Mp) from a single reaction is less than the mass of the original fuel nucleus (M). The excess mass őĒm = M - Mp is the invariant mass of the energy that is released as photons (gamma rays) and kinetic energy of the fission fragments, according to the mass-energy equivalence formula, E¬†=¬†mc¬≤.
In nuclear fission events, the nuclei may break into any combination of lighter nuclei, but the most common event is not fission to equal mass nuclei of about mass 120; the most common event (depending on isotope and process) is a slightly unequal fission in which one daughter nucleus has a mass of about 90 to 100 u and the other, the remaining 130 to 140 u. Unequal fissions are energetically more favorable because this allows one product to be closer to the energetic minimum near mass 60 u (only a quarter of the average fissionable mass), while the other nucleus with mass 135 u is still not far out of the range of the most tightly bound nuclei (another statement of this is that the atomic binding energy curve is slightly steeper to the left of mass 120 u than to the right of it).
The variation in specific binding energy with atomic number is due to the interplay of the two fundamental forces acting on the component nucleons (protons and neutrons) that make up the nucleus. Nuclei are bound by an attractive strong nuclear force between nucleons, which overcomes the electrostatic repulsion between protons. However, the strong nuclear force acts only over extremely short ranges, since it follows a Yukawa potential. For this reason, large nuclei are less tightly bound per unit mass than small nuclei, and breaking a very large nucleus into two or more intermediate-sized nuclei releases energy.
Because of the short range of the strong binding force, large nuclei must contain proportionally more neutrons than light elements do, which are most stable with a 1-1 ratio of protons and neutrons. Extra neutrons stabilize heavy elements because they add to strong-force binding without adding to proton-proton repulsion. Fission products have, on average, about the same ratio of neutrons and protons as their parent nucleus, and are therefore usually unstable because they have proportionally too many neutrons compared to stable isotopes of similar mass. This is the fundamental cause of the problem of radioactive high level waste from nuclear reactors. Fission products tend to be beta emitters, emitting fast-moving electrons to conserve electric charge as excess neutrons convert to protons inside the nucleus of the fission product atoms.
The most common nuclear fuels, 235U and 239Pu, are not major radiologic hazards by themselves: 235U has a half-life of approximately 700 million years, and although 239Pu has a half-life of only about 24,000 years, it is a pure alpha particle emitter and, hence, not particularly dangerous unless ingested. Once a fuel element has been used, the remaining fuel material is intimately mixed with highly radioactive fission products that emit energetic beta particles and gamma rays. Some fission products have half-lives as short as seconds; others have half-lives of tens of thousands of years, requiring long-term storage in facilities such as Yucca mountain until the fission products decay into non-radioactive stable isotopes.
Chain reactions

Many heavy elements, such as uranium, thorium, and plutonium, undergo both spontaneous fission, a form of radioactive decay, and induced fission, a form of nuclear reaction. Elemental isotopes that undergo induced fission when struck by a free neutron are called fissionable; isotopes that undergo fission when struck by a thermal, slow moving neutron are also called fissile. A few particularly fissile and readily obtainable isotopes (notably 235U and 239Pu) are called nuclear fuels because they can sustain a chain reaction and can be obtained in large enough quantities to be useful.
All fissionable and fissile isotopes undergo a small amount of spontaneous fission which releases a few free neutrons into any sample of nuclear fuel. Such neutrons escape rapidly from the fuel and become known as free neutrons, with a half-life of about 15 minutes before they decayed to protons and beta particles. However, neutrons almost invariably impact and are absorbed by other nuclei in the vicinity long before this happens (newly-created fission neutrons are moving at about 7 percent of the speed of light, and even moderated neutrons are moving at about 8 times the speed of sound). Some neutrons will impact fuel nuclei and induce further fissions, releasing yet more neutrons. If enough nuclear fuel is assembled into one place, or if the escaping neutrons are sufficiently contained, then these freshly generated neutrons outnumber the neutrons that escape from the assembly, and a sustained nuclear chain reaction will take place.
An assembly that supports a sustained nuclear chain reaction is called a critical assembly or, if the assembly is almost entirely made of a nuclear fuel, a critical mass. The word "critical" refers to a cusp in the behavior of the differential equation that governs the number of free neutrons present in the fuel: If less than a critical mass is present, then the amount of neutrons is determined by radioactive decay, but if a critical mass or more is present, then the amount of neutrons is controlled instead by the physics of the chain reaction. The actual mass of a critical mass of nuclear fuel depends strongly on the geometry and surrounding materials.
Not all fissionable isotopes can sustain a chain reaction. For example, 238U, the most abundant form of uranium, is fissionable but not fissile: It undergoes induced fission when impacted by an energetic neutron with over 1 MeV of kinetic energy. But too few of the neutrons produced by 238U fission are energetic enough to induce further fissions in 238U, so no chain reaction is possible with this isotope. Instead, bombarding 238U with slow neutrons causes it to absorb them (becoming 239U) and decay by beta emission to 239Np which then decays again by the same process to 239Pu; that process is used to manufacture 239Pu in breeder reactors, but does not contribute to a neutron chain reaction.
Fissionable, non-fissile isotopes can be used as fission energy source even without a chain reaction. Bombarding 238U with fast neutrons induces fissions, releasing energy as long as the external neutron source is present. That effect is used to augment the energy released by modern thermonuclear weapons, by jacketing the weapon with 238U to react with neutrons released by nuclear fusion at the center of the device.
Fission reactors
Critical fission reactors are the most common type of nuclear reactor. In a critical fission reactor, neutrons produced by fission of fuel atoms are used to induce yet more fissions, to sustain a controllable amount of energy release. Devices that produce engineered but non-self-sustaining fission reactions are subcritical fission reactors. Such devices use radioactive decay or particle accelerators to trigger fissions.
Critical fission reactors are built for three primary purposes, which typically involve different engineering trade-offs to take advantage of either the heat or the neutrons produced by the fission chain reaction:
- Power reactors are intended to produce heat for nuclear power, either as part of a generating station or a local power system such as in a nuclear submarine.
- Research reactors are intended to produce neutrons and/or activate radioactive sources for scientific, medical, engineering, or other research purposes.
- Breeder reactors are intended to produce nuclear fuels in bulk from more abundant isotopes. The better known fast breeder reactor makes 239Pu (a nuclear fuel) from the naturally very abundant 238U (not a nuclear fuel). Thermal breeder reactors previously tested using 232Th continue to be studied and developed.
While, in principle, all fission reactors can act in all three capacities, in practice the tasks lead to conflicting engineering goals and most reactors have been built with only one of the above tasks in mind. (There are several early counter-examples, such as the Hanford N reactor, now decommissioned.) Power reactors generally convert the kinetic energy of fission products into heat, which is used to heat a working fluid and drive a heat engine that generates mechanical or electrical power. The working fluid is usually water with a steam turbine, but some designs use other materials, such as gaseous helium. Research reactors produce neutrons that are used in various ways, with the heat of fission being treated as an unavoidable waste product. Breeder reactors are a specialized form of research reactor, with the caveat that the sample being irradiated is usually the fuel itself, a mixture of 238U and 235U.
Fission bombs
One class of nuclear weapon, a fission bomb (not to be confused with the fusion bomb), otherwise known as an atomic bomb or atom bomb, is a fission reactor designed to liberate as much energy as possible as rapidly as possible, before the released energy causes the reactor to explode (and the chain reaction to stop). Development of nuclear weapons was the motivation behind early research into nuclear fission: The Manhattan Project of the U.S. military during World War II carried out most of the early scientific work on fission chain reactions, culminating in the Little Boy and Fat Man and Trinity bombs that were exploded over test sites, the cities Hiroshima, and Nagasaki, Japan, in August of 1945.
Even the first fission bombs were thousands of times more explosive than a comparable mass of chemical explosive. For example, Little Boy weighed a total of about four tons (of which 60 kg was nuclear fuel) and was 11 feet long; it also yielded an explosion equivalent to about 15,000 tons of TNT, destroying a large part of the city of Hiroshima. Modern nuclear weapons (which include a thermonuclear fusion as well as one or more fission stages) are literally hundreds of times more energetic for their weight than the first pure fission atomic bombs, so that a modern single missile warhead bomb weighing less than 1/8th as much as Little Boy (see for example W88) has a yield of 475,000 tons of TNT, and could bring destruction to 10 times the city area.
While the fundamental physics of the fission chain reaction in a nuclear weapon is similar to the physics of a controlled nuclear reactor, the two types of device must be engineered quite differently. It would be extremely difficult to convert a nuclear reactor to cause a true nuclear explosion (though partial fuel meltdowns and steam explosions have occurred), and similarly difficult to extract useful power from a nuclear explosive (though at least one rocket propulsion system, Project Orion, was intended to work by exploding fission bombs behind a massively padded vehicle).
The strategic importance of nuclear weapons is a major reason why the technology of nuclear fission is politically sensitive. Viable fission bomb designs are within the capabilities of bright undergraduates (see John Aristotle Phillips) being incredibly simple, but nuclear fuel to realize the designs is thought to be difficult to obtain being rare (see uranium enrichment and nuclear fuel cycle).
History
In 1919, Ernest Rutherford became the first person to deliberately split the atom by bombarding nitrogen with naturally occurring alpha particles from radioactive material and observing a proton emitted with energy higher than the alpha particle. In 1932, John Cockcroft and Ernest Walton, working under Rutherford`s direction, first split the nucleus by entirely artificial means, using a particle accelerator to bombard lithium with protons thereby producing two alpha particles.[1]
Results of the bombardment of uranium by neutrons had proved interesting and puzzling. First studied by Enrico Fermi and his colleagues in 1934, they were not properly interpreted until several years later.
After the Fermi publication, Lise Meitner, Otto Hahn, and Fritz Strassmann began performing similar experiments in Germany. Meitner, an Austrian Jew, lost her citizenship with the Anschluss in 1938. She fled and wound up in Sweden, but continued to collaborate by mail and through meetings with Hahn in Sweden. By coincidence her nephew Otto Robert Frisch, also a refugee, was also in Sweden when Meitner received a letter from Hahn describing his chemical proof that some of the product of the bombardment of uranium with neutrons was barium (barium's atomic weight is half that of uranium). Frisch was skeptical, but Meitner believed Hahn was too good a chemist to have made a mistake. According to Frisch:
Was it a mistake? No, said Lise Meitner; Hahn was too good a chemist for that. But how could barium be formed from uranium? No larger fragments than protons or helium nuclei (alpha particles) had ever been chipped away from nuclei, and to chip off a large number not nearly enough energy was available. Nor was it possible that the uranium nucleus could have been cleaved right across. A nucleus was not like a brittle solid that can be cleaved or broken; George Gamow had suggested early on, and Bohr had given good arguments that a nucleus was much more like a liquid drop. Perhaps a drop could divide itself into two smaller drops in a more gradual manner, by first becoming elongated, then constricted, and finally being torn rather than broken in two? We knew that there were strong forces that would resist such a process, just as the surface tension of an ordinary liquid drop tends to resist its division into two smaller ones. But nuclei differed from ordinary drops in one important way: They were electrically charged, and that was known to counteract the surface tension.
The charge of a uranium nucleus, we found, was indeed large enough to overcome the effect of the surface tension almost completely; so the uranium nucleus might indeed resemble a very wobble unstable drop, ready to divide itself at the slightest provocation, such as the impact of a single neutron. But there was another problem. After separation, the two drops would be driven apart by their mutual electric repulsion and would acquire high speed and hence a very large energy, about 200 MeV in all; where could that energy come from? …Lise Meitner… worked out that the two nuclei formed by the division of a uranium nucleus together would be lighter than the original uranium nucleus by about one-fifth the mass of a proton. Now whenever mass disappears energy is created, according to Einstein's formula E=mc2, and one-fifth of a proton mass was just equivalent to 200MeV. So here was the source for that energy; it all fitted!
The basic discovery and chemical proof of Otto Hahn and Fritz Strassmann that an isotope of barium was produced by neutron bombardment of uranium was published in a paper in Germany in the Journal Naturwissenschaften, January 6, 1939) and earned Hahn a Nobel Prize.[2]
Frisch rapidly confirmed, experimentally, by means of a cloud chamber, that the uranium atom had indeed been split by the action of neutrons. A fundamental idea of this experiment was suggested to Frisch by George Placzek.[3]
Two papers were mailed to England on January 16, 1939, the first on the interpretation of the barium appearance as atom splitting by Meitner and Frisch, the second on the experimental confirmation by Frisch (strangely omitting Placzek's important contribution, however). The first paper appeared on February 11, the second on February 28.[4]
Meitner and Frisch's theory and mathematical proof of Hahn's discovery and chemical proof of barium products from the bombardment of uranium was the foundation of the later research on nuclear fission. The awarding of the 1944 Nobel Prize in Chemistry to Hahn alone is a longstanding controversy.[5]
On January 16, 1939, Niels Bohr of Copenhagen, Denmark, arrived in the United States, to spend several months in Princeton, New Jersey, and was particularly anxious to discuss some abstract problems with Albert Einstein. (Four years later, Bohr was to escape to Sweden from Nazi-occupied Denmark in a small boat, along with thousands of other Danish Jews, in large scale operation.) Just before Bohr left Denmark, Frisch and Meitner gave him their calculations.
Bohr had promised to keep the Meitner/Frisch paper secret until it was published to preserve priority, but on the boat he discussed it with Léon Rosenfeld, and forgot to tell him to keep it secret. Rosenfeld immediately upon arrival told everyone at Princeton University, and from them the news spread by word of mouth to neighboring physicists including Enrico Fermi at Columbia University. Fermi upon traveling to receive the Nobel Prize for his earlier work. headed to the USA rather than return to Fascist Italy with his Jewish wife. As a result of conversations among Fermi, John R. Dunning, and G. B. Pegram, a search was undertaken at Columbia for the heavy pulses of ionization that would be expected from the flying fragments of the uranium nucleus. On January 26, 1939, there was a conference on theoretical physics at Washington, D.C., sponsored jointly by the George Washington University and the Carnegie Institution of Washington. Before the meeting in Washington was over, several other experiments to confirm fission had been initiated, and positive experimental confirmation was reported.
Frédéric Joliot-Curie's team in Paris discovered that secondary neutrons are released during uranium fission thus making a chain reaction feasible. About two neutrons being emitted with nuclear fission of uranium was verified independently by Leo Szilard and Walter Zinn. The number of neutrons emitted with nuclear fission of 235uranium was then reported at 3.5/fission, and later corrected to 2.6/fission by Frédéric Joliot-Curie, Hans von Halban and Lew Kowarski.
"Chain reactions" at that time were a known phenomenon in chemistry but the analogous process in nuclear physics using neutrons had been foreseen as early as 1933 by Leo Szilard, although Szilard at that time had no idea with what materials the process might be initiated. Szilard, a Hungarian born Jew, also fled mainland Europe after Hitler's rise, eventually landing in the U.S.
In the summer, Fermi and Szilard proposed the idea of a nuclear reactor (pile) with natural uranium as fuel and graphite as moderator of neutron energy.
In August, Hungarian-Jewish refugees Szilard, Teller, and Wigner persuaded Austrian-Jewish refugee Einstein to warn President Roosevelt of the German menace. The letter suggested the possibility of uranium bomb deliverable by ship. The President received it on October 11, 1939, shortly after World War II began.
In England, James Chadwick proposed an atomic bomb utilizing natural uranium based on a paper by Rudolf Peierls, with the mass needed for critical state being 30-40 tons.
In December, Heisenberg delivered a report to the Germany Department of War on the possibility of a uranium bomb.
In Birmingham, England, Otto Robert Frisch teamed up with Rudolf Peierls who had also fled German anti-Jewish race laws. They conceived the idea of utilizing a purified isotope of uranium, uranium-235, and worked out that an enriched uranium bomb could have a critical mass of only 600 g, instead of tons, and that the resulting explosion would be tremendous (the amount actually turned out to be 15 kg). In February 1940, they delivered the Frisch-Peierls memorandum, however, they were officially considered "enemy aliens" at the time.
Uranium-235 was separated by Nier and fission with slow neutron was confirmed by Dunning.
German-Jewish refugee Francis Simon, at Oxford, quantified the gaseous diffusion separation of U-235.
In 1941, American Physicist Ernest O. Lawrence proposed electromagnetic separation.
Glenn Seaborg, Joe Kennedy, Art Wahl, and Italian-Jewish refugee Emilio Segre discovered plutonium and determined it to be fissionable, like U-235. (Lawrence controversially dropped Segre's pay by half when he learned he was trapped in the U.S. by Mussolini's race laws.)
On June 28, 1941, the Office of Scientific Research and Development was formed to mobilize scientific resources and apply the results of research to national defense. In September, Fermi assembled his first nuclear pile in an attempt to create a slow neutron induced chain reaction in uranium, but the experiment failed.
Producing a fission chain reaction in uranium fuel is far from trivial. Early nuclear reactors did not use isotopically enriched uranium, and in consequence they were required to use large quantities of highly purified graphite as neutron moderation materials. Use of ordinary water (as opposed to heavy water) in nuclear reactors requires enriched fuel‚ÄĒthe partial separation and relative enrichment of the rare 235U isotope from the far more common 238U isotope. Typically, reactors also require inclusion of extremely chemically pure neutron moderator materials such as deuterium (in heavy water), helium, beryllium, or carbon, usually as the graphite. (The high purity is required because many chemical impurities such as the boron-10 component of natural boron, are very strong neutron absorbers and thus poison the chain reaction.)
Production of such materials at industrial scale had to be solved for nuclear power generation and weapons production to be accomplished. Up to 1940, the total amount of uranium metal produced in the U.S. was not more than a few grams, and even this was of doubtful purity; of metallic beryllium not more than a few kilograms; concentrated deuterium oxide (heavy water) not more than a few kilograms; and finally carbon had never been produced in quantity with anything like the purity required of a moderator.
The problem of producing large amounts of high purity uranium was solved by Frank Spedding using the thermite process. Ames Laboratory was established in 1942, to produce the large amounts of natural (unenriched) uranium that would be necessary for the research to come. The success of the Chicago Pile-1 which used unenriched (natural) uranium, like all of the atomic "piles" which produced the plutonium for the atomic bomb, was also due specifically to Szilard's realization that very pure graphite could be used for the moderator of even natural uranium "piles." In wartime Germany, failure to appreciate the qualities of very pure graphite led to reactor designs dependent on heavy water, which in turn was denied the Germans by allied attacks in Norway, where heavy water was produced. These difficulties prevented the Nazis from building a nuclear reactor capable of criticality during the war.
Unknown until 1972 (but postulated by Paul Kuroda in 1956), when French physicist Francis Perrin discovered the Oklo Fossil Reactors, nature had beaten humans to the punch by engaging in large-scale uranium fission chain reactions, some 2,000 million years in the past. This ancient process was able to use normal water as a moderator, only because 2,000 million years in the past, natural uranium was "enriched" with the shorter-lived fissile isotope 235U, as compared with the natural uranium available today.
For more detail on the early development of nuclear reactors and nuclear weapons, see Manhattan Project.
Notes
- ‚ÜĎ Rutherford Mythology, Splitting the atom.
- ‚ÜĎ Otto Hahn, Fritz Strassmann, 1939, √úber den Nachweis und das Verhalten der bei der Bestrahlung des Urans mittels Neutronen entstehenden Erdalkalimetalle. Naturwissenschaften. 27:1:11-15.
- ‚ÜĎ O.R. Frisch, The Discovery of Fission‚ÄĒHow It All Began, Physics Today, (1967) 20:11:43-48.
- ‚ÜĎ Lise Meitner and Otto Robert Frisch, Disintegration of Uranium by Neutrons: a New Type of Nuclear Reaction, Nature (1939) 143:239-240.
- ‚ÜĎ Alfred Neubauer, Bittere Nobelpreise (Noderstedt, DE: Book on Demand, 2005). ISBN 3-8334-3448-1
ReferencesISBN links support NWE through referral fees
- Krane, Kenneth S. and David Halliday. 1988. Introductory Nuclear Physics. New York: Wiley. ISBN 047180553X
- Martin, Brian. 2006. Nuclear and Particle Physics: An Introduction. Hoboken, NJ: Wiley. ISBN 0470025328
- Poenaru, D. N. 1996. Nuclear Decay Modes. Fundamental and Applied Nuclear Physics Series. Philadelphia: Institute of Physics. ISBN 0750303387
- Tipler, Paul and Ralph Llewellyn. 2002. Modern Physics. New York: W.H. Freeman. ISBN 0-7167-4345-0
- Turner, James E. 1995. Atoms, Radiation, and Radiation Protection. New York: Wiley. ISBN 0471595810
External links
All links retrieved July 31, 2025.
- The Effects of Nuclear Weapons.
- Nuclear Fission Explained. Atomicarchive.com.
- Nuclear Fission Animation. Atomicarchive.com.
| Nuclear technology | |
|---|---|
| Nuclear engineering | Nuclear physics | Nuclear fission | Nuclear fusion | Radiation | Ionizing radiation | Atomic nucleus | Nuclear reactor | Nuclear safety |
| Nuclear material | Nuclear fuel | Fertile material | Thorium | Uranium | Enriched uranium | Depleted uranium | Plutonium |
| Nuclear power | Nuclear power plant | Radioactive waste | Fusion power | Future energy development | Inertial fusion power plant | Pressurized water reactor | Boiling water reactor | Generation IV reactor | Fast breeder reactor | Fast neutron reactor | Magnox reactor | Advanced gas-cooled reactor | Gas-cooled fast reactor | Molten salt reactor | Liquid-metal-cooled reactor | Lead-cooled fast reactor | Sodium-cooled fast reactor | Supercritical water reactor | Very high temperature reactor | Pebble bed reactor | Integral Fast Reactor | Nuclear propulsion | Nuclear thermal rocket | Radioisotope thermoelectric generator |
| Nuclear medicine | PET | Radiation therapy | Tomotherapy | Proton therapy | Brachytherapy |
| Nuclear weapons | History of nuclear weapons | Nuclear warfare | Nuclear arms race | Nuclear weapon design | Effects of nuclear explosions | Nuclear testing | Nuclear delivery | Nuclear proliferation | List of states with nuclear weapons | List of nuclear tests |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.