Difference between revisions of "Sodium hydroxide" - New World Encyclopedia
Rosie Tanabe (talk | contribs) |
|||
| (9 intermediate revisions by 5 users not shown) | |||
| Line 1: | Line 1: | ||
| − | + | {{Images OK}}{{Submitted}}{{Approved}}{{Paid}}{{copyedited}} | |
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
! {{chembox header}} | Sodium hydroxide <!-- replace if not identical with the article name —> | ! {{chembox header}} | Sodium hydroxide <!-- replace if not identical with the article name —> | ||
| Line 49: | Line 49: | ||
|- | |- | ||
| [[Material safety data sheet|MSDS]] | | [[Material safety data sheet|MSDS]] | ||
| − | | | + | | External MSDS |
|- | |- | ||
| [[Directive 67/548/EEC|EU classification]] | | [[Directive 67/548/EEC|EU classification]] | ||
| Line 91: | Line 91: | ||
| | | | ||
|- | |- | ||
| − | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br> materials in their [[standard state|standard state (at 25 °C, 100 kPa) | + | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br/> materials in their [[standard state|standard state (at 25 °C, 100 kPa)]]</small> |
|- | |- | ||
|} | |} | ||
| − | '''Sodium hydroxide''' | + | '''Sodium hydroxide,''' also known as '''lye''' or '''caustic soda,''' is a [[caustic (substance)|caustic]] metallic [[Base (chemistry)|base]]. Its [[chemical formula]] is [[sodium|Na]][[hydroxide|OH]]. Forming a strongly [[alkaline]] solution when dissolved in a solvent such as water, caustic soda is widely used in many industries, mostly as a strong [[chemical compound|chemical]] [[pH|base]] in the manufacture of [[pulp]] and [[paper]], [[textile]]s, [[drinking water]], [[soap]]s, and [[detergent]]s. Worldwide production in 1998, was around 45 million [[ton]]s. Sodium hydroxide is also the most common base used in chemical laboratories, and it is widely used as a drain cleaner. |
| − | + | {{toc}} | |
== General properties == | == General properties == | ||
| − | + | Pure sodium hydroxide is a white solid; available in pellets, flakes, granules, and also as a 50-percent saturated solution. It is [[deliquescent]] and also readily absorbs [[carbon dioxide]] from the air, so it should be stored in an [[Hermetically sealed|airtight]] container. It is very soluble in water, with liberation of heat. It also dissolves in [[ethanol]] and [[methanol]], though it exhibits lower solubility in these solvents than does [[potassium hydroxide]]. It is insoluble in [[diethyl ether|ether]] and other non-polar solvents. A sodium hydroxide [[solution]] will leave a yellow stain on fabric and paper. | |
| − | Pure sodium hydroxide is a white solid; available in pellets, flakes, granules, and also as a 50-percent saturated solution. It is [[deliquescent]] and also readily absorbs [[carbon dioxide]] from the air, so it should be stored in an [[Hermetically sealed|airtight]] container. It is very soluble in water, with liberation of heat. It also dissolves in [[ethanol]] and [[methanol]], though it exhibits lower solubility in these solvents than does [[potassium hydroxide]]. It is insoluble in [[diethyl ether|ether]] and other | ||
== Chemical properties == | == Chemical properties == | ||
| − | + | Sodium hydroxide is completely [[Ionic bonding|ionic]], containing sodium ions and [[hydroxide]] ions. The hydroxide ion makes sodium hydroxide a strong base which reacts with acids to form [[water]] and the corresponding salts, for example, with [[hydrochloric acid]], [[sodium chloride]] is formed: | |
| − | Sodium hydroxide is completely [[Ionic bonding|ionic]], containing sodium ions and [[hydroxide]] ions. | ||
:NaOH([[Aqueous|aq]]) + [[Hydrochloric acid|HCl]](aq) → [[Sodium chloride|NaCl]](aq) + [[Water (molecule)|H<sub>2</sub>O]]([[Liquid|l]]) | :NaOH([[Aqueous|aq]]) + [[Hydrochloric acid|HCl]](aq) → [[Sodium chloride|NaCl]](aq) + [[Water (molecule)|H<sub>2</sub>O]]([[Liquid|l]]) | ||
| − | In general such [[neutralization]] reactions are represented by one simple net ionic equation: | + | In general, such [[neutralization]] reactions are represented by one simple net ionic equation: |
:[[Hydroxide|OH<sup>−</sup>]](aq) + [[Hydronium|H<sup>+</sup>(aq)]] → H<sub>2</sub>O | :[[Hydroxide|OH<sup>−</sup>]](aq) + [[Hydronium|H<sup>+</sup>(aq)]] → H<sub>2</sub>O | ||
| − | This type of reaction [[exothermic reaction|releases heat]] when a strong acid is used. | + | This type of reaction [[exothermic reaction|releases heat]] when a strong acid is used. Such [[acid-base reaction]]s can also be used for [[titration]]s, and indeed this is a common way for measuring the concentration of acids. |
| − | Related to this is the reaction of sodium hydroxide with acidic oxides. The reaction of [[carbon dioxide]] has already been mentioned, but other acidic oxides such as [[sulfur dioxide]] (SO<sub>2</sub>) also react completely. | + | |
| + | Related to this is the reaction of sodium hydroxide with acidic oxides. The reaction of [[carbon dioxide]] has already been mentioned, but other acidic oxides such as [[sulfur dioxide]] (SO<sub>2</sub>) also react completely. Such reactions are often used to "scrub" harmful acidic gases (like SO<sub>2</sub> and H<sub>2</sub>S) and prevent their release into the atmosphere. | ||
:2NaOH + [[Carbon dioxide|CO<sub>2</sub>]] → [[Sodium carbonate|Na<sub>2</sub>CO<sub>3</sub>]] + H<sub>2</sub>O | :2NaOH + [[Carbon dioxide|CO<sub>2</sub>]] → [[Sodium carbonate|Na<sub>2</sub>CO<sub>3</sub>]] + H<sub>2</sub>O | ||
| − | Sodium hydroxide slowly reacts with glass to form [[sodium silicate]], so glass joints and [[stopcock]]s exposed to NaOH have a tendency to "freeze" | + | Sodium hydroxide slowly reacts with glass to form [[sodium silicate]], so glass joints and [[stopcock]]s exposed to NaOH have a tendency to "freeze." [[Laboratory flask|Flask]]s and glass-lined [[chemical reactor]]s are damaged by long exposure to hot sodium hydroxide, and the glass becomes frosted. Sodium hydroxide does not attack [[iron]] or [[copper]], but many other metals such as [[aluminium]], [[zinc]], and [[titanium]] are attacked rapidly. In 1986, an aluminum [[tank truck|road tanker]] in the UK was mistakenly used to transport 25 percent sodium hydroxide solution, causing pressurization of the contents and damage to the tanker. For this same reason aluminum pans should never be cleaned with lye. |
:2[[Aluminium|Al]]([[Solid|s]]) + 6NaOH(aq) → 3[[Hydrogen|H<sub>2</sub>]]([[Gas|g]]) + 2Na<sub>3</sub>AlO<sub>3</sub>(aq) | :2[[Aluminium|Al]]([[Solid|s]]) + 6NaOH(aq) → 3[[Hydrogen|H<sub>2</sub>]]([[Gas|g]]) + 2Na<sub>3</sub>AlO<sub>3</sub>(aq) | ||
| − | Many non-metals also react with sodium hydroxide, giving salts. For example [[phosphorus]] forms [[sodium hypophosphite]], while [[silicon]] gives [[sodium silicate]]. | + | Many non-metals also react with sodium hydroxide, giving salts. For example, [[phosphorus]] forms [[sodium hypophosphite]], while [[silicon]] gives [[sodium silicate]]. |
| − | Unlike NaOH, the hydroxides of most metals are insoluble, and therefore sodium hydroxide can be used to precipitate metal hydroxides. One such hydroxide is [[aluminium hydroxide]], used as a gelatinous [[flocculation|floc]] to filter out particulate matter in [[water treatment]]. | + | Unlike NaOH, the hydroxides of most metals are insoluble, and therefore sodium hydroxide can be used to precipitate metal hydroxides. One such hydroxide is [[aluminium hydroxide]], used as a gelatinous [[flocculation|floc]] to filter out particulate matter in [[water treatment]]. Aluminum hydroxide is prepared at the treatment plant from [[aluminum sulfate]] by reaction with NaOH: |
| − | :6NaOH(aq) | + | :6NaOH(aq) + [[Aluminium sulfate|Al<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub>]](aq) → 2[[Aluminium hydroxide|Al(OH)<sub>3</sub>]](s) + 3[[Sodium sulfate|Na<sub>2</sub>SO<sub>4</sub>]](aq) |
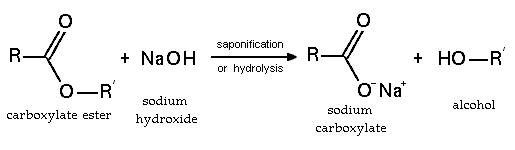
Sodium hydroxide reacts readily with [[carboxylic acid]]s to form their salts, and it is even a strong enough base to form salts with [[phenol]]s. NaOH can also be used for the base-driven [[hydrolysis]] of [[ester]]s (as is [[saponification]]), [[amide]]s and [[alkyl halide]]s. However, the limited solubility of NaOH in organic solvents means that the more [[soluble]] [[Potassium hydroxide|KOH]] is often preferred. | Sodium hydroxide reacts readily with [[carboxylic acid]]s to form their salts, and it is even a strong enough base to form salts with [[phenol]]s. NaOH can also be used for the base-driven [[hydrolysis]] of [[ester]]s (as is [[saponification]]), [[amide]]s and [[alkyl halide]]s. However, the limited solubility of NaOH in organic solvents means that the more [[soluble]] [[Potassium hydroxide|KOH]] is often preferred. | ||
| Line 131: | Line 130: | ||
== Manufacture == | == Manufacture == | ||
| − | In 1998, total world production was around 45 million [[ | + | In 1998, total world production was around 45 million [[ton]]s. Of this, both North America and Asia contributed around 14 million metric tons, and Europe produced around 10 million metric tons. |
=== Methods of production=== | === Methods of production=== | ||
| − | Sodium hydroxide is produced (along with [[chlorine]] and [[hydrogen]]) via the [[chloralkali process]]. | + | Sodium hydroxide is produced (along with [[chlorine]] and [[hydrogen]]) via the [[chloralkali process]]. This involves the [[electrolysis]] of an aqueous solution of [[sodium chloride]]. The sodium hydroxide builds up at the [[cathode]], where water is reduced to hydrogen gas and [[hydroxide]] ion: |
:2Na<sup>+</sup> + 2H<sub>2</sub>O + 2e<sup>−</sup> → H<sub>2</sub> + 2NaOH | :2Na<sup>+</sup> + 2H<sub>2</sub>O + 2e<sup>−</sup> → H<sub>2</sub> + 2NaOH | ||
| − | To produce NaOH it is necessary to prevent reaction of the NaOH with the [[chlorine]]. | + | To produce NaOH, it is necessary to prevent reaction of the NaOH with the [[chlorine]]. This is typically done in one of three ways, of which the membrane cell process is economically the most viable. |
| − | * '''Mercury cell process''' (also called the [[Castner-Kellner process]]) | + | * '''Mercury cell process''' (also called the [[Castner-Kellner process]])—[[Sodium|sodium metal]] forms as an [[amalgam]] at a [[mercury (element)|mercury]] [[cathode]]; this sodium is then reacted with water to produce NaOH. There have been concerns about mercury releases, although modern plants claim to be safe in this regard.<ref>Euro Chlor, [http://www.eurochlor.org/animations/mercury-cell.asp Chlorine Online Diagram of mercury cell process.] Retrieved April 4, 2008.</ref> |
| − | * '''Diaphragm cell process''' | + | * '''Diaphragm cell process'''—uses a steel cathode, and reaction of NaOH with Cl<sub>2</sub> is prevented using a porous [[diaphragm seal|diaphragm]]. In the diaphragm cell process, the anode area is separated from the cathode area by a permeable diaphragm. The brine is introduced into the anode compartment and flows through the diaphragm into the cathode compartment. A diluted caustic brine leaves the cell. The caustic soda must usually be concentrated to 50 percent and the salt removed. This is done using an evaporative process with about three metric tons of steam per metric ton of caustic soda. The salt separated from the caustic brine can be used to saturate diluted brine. The chlorine contains oxygen and must often be purified by liquefaction and evaporation.<ref>Euro Chlor, [http://www.eurochlor.org/makingchlorine How is chlorine made?] Retrieved April 4, 2008.</ref> |
| − | * '''Membrane cell process''' | + | * '''Membrane cell process'''—similar to the diaphragm cell process, with a [[Nafion]] membrane to separate the cathode and anode reactions. Only sodium ions and a little water pass through the membrane. It produces a higher quality of NaOH. Of the three processes, the membrane cell process requires the lowest consumption of electric energy and the amount of steam needed for concentration of the caustic is relatively small (less than one metric ton per metric ton of caustic soda).<ref>Euro Chlor, [http://www.eurochlor.org/animations/membrane-cell.asp Chlorine Online Diagram of membrane cell process.] Retrieved April 4, 2008.</ref> |
| − | An older method for sodium hydroxide production was the [[LeBlanc process]], which produced [[sodium carbonate]], followed by roasting to create [[carbon dioxide]] and [[sodium oxide]]. This method is still occasionally used. | + | An older method for sodium hydroxide production was the [[LeBlanc process]], which produced [[sodium carbonate]], followed by roasting, to create [[carbon dioxide]] and [[sodium oxide]]. This method is still occasionally used. It helped to establish sodium hydroxide as an important commodity chemical. |
=== Major producers === | === Major producers === | ||
| − | In the United States, the major producer of sodium hydroxide is the [[Dow Chemical Company]], which has annual production around 3.7 million [[ | + | In the United States, the major producer of sodium hydroxide is the [[Dow Chemical Company]], which has annual production around 3.7 million [[ton]]s from sites at [[Freeport, Texas]], and [[Plaquemine, Louisiana]]. Other major U.S. producers include [[Oxychem]], [[PPG Industries|PPG]], [[Olin Corporation|Olin]], Pioneer Companies, Inc. (PIONA), and [[Formosa Plastics Group|Formosa]]. All of these companies use the [[chloralkali process]].<ref>''Kirk-Othmer Encyclopedia of Chemical Technology.''</ref> |
== Uses == | == Uses == | ||
=== General applications === | === General applications === | ||
| − | Sodium hydroxide is the principal strong [[base (chemistry)|base]] used in the chemical industry. In bulk it is most often handled as an [[aqueous]] [[solution]], since solutions are cheaper and easier to handle. | + | Sodium hydroxide is the principal strong [[base (chemistry)|base]] used in the chemical industry. In bulk, it is most often handled as an [[aqueous]] [[solution]], since solutions are cheaper and easier to handle. It is used to drive for chemical reactions and also for the [[neutralization]] of acidic materials. It can be used also as a neutralizing agent in petroleum refining. |
===Gold pennies=== | ===Gold pennies=== | ||
| − | Sodium hydroxide has also been used in conjunction with [[zinc]] for creation of the famous "Gold pennies" experiment. | + | Sodium hydroxide has also been used in conjunction with [[zinc]] for creation of the famous "Gold pennies" experiment. When a [[penny]] is boiled in a solution of NaOH together with some granular zinc metal ([[Hot-dip galvanizing|galvanized nails]] are one source), the color of the penny will turn silver in about 45 seconds. The penny is then held in the flame of a burner for a few seconds and it turns golden. The reason this happens is that granular zinc dissolves in NaOH to form Zn(OH)<sub>4</sub><sup>2-</sup>. This zincate ion becomes reduced to metallic zinc on the surface of a [[copper]] penny. Zinc and copper when heated in a flame form [[brass]]. |
=== Use in chemical analysis === | === Use in chemical analysis === | ||
| − | In [[analytical chemistry]], sodium hydroxide solutions are often used to measure the [[concentration]] of acids by [[titration]]. | + | In [[analytical chemistry]], sodium hydroxide solutions are often used to measure the [[concentration]] of acids by [[titration]]. Since NaOH is not a [[primary standard]], solutions must first be standardized by titration against a standard such as [[Potassium hydrogen phthalate|KHP]]. [[Burette]]s exposed to NaOH should be rinsed out immediately after use to prevent "freezing" of the stopcock. Sodium hydroxide was traditionally used to test for [[cation]]s in [[Qualitative inorganic analysis|Qualitative Inorganic Analysis]], as well as to provide alkaline media for some reactions that need it, such as the [[Biuret]] test. |
=== Soap making === | === Soap making === | ||
| Line 167: | Line 166: | ||
=== Aluminum etching === | === Aluminum etching === | ||
| − | Strong bases attack [[ | + | Strong bases attack [[aluminum]]. This can be useful in etching through a resist or in converting a polished surface to a satin-like finish, but without further [[passivation]] such as [[anodizing]] or [[allodizing]] the surface may become corroded, either under normal use or in severe atmospheric conditions. |
=== Food preparation === | === Food preparation === | ||
| Line 173: | Line 172: | ||
Specific foods processed with lye include: | Specific foods processed with lye include: | ||
| − | * The [[Scandinavia]]n delicacy known as [[lutefisk]] (from ''lutfisk'' | + | * The [[Scandinavia]]n delicacy known as [[lutefisk]] (from ''lutfisk,'' "lye fish"). |
* [[Hominy]] is dried [[maize]] (corn) kernels reconstituted by soaking in lye-water. These expand considerably in size and may be further processed by cooking in hot oil and salting to form [[corn nuts]]. [[Nixtamal]] is similar, but uses [[calcium hydroxide]] instead of sodium hydroxide. | * [[Hominy]] is dried [[maize]] (corn) kernels reconstituted by soaking in lye-water. These expand considerably in size and may be further processed by cooking in hot oil and salting to form [[corn nuts]]. [[Nixtamal]] is similar, but uses [[calcium hydroxide]] instead of sodium hydroxide. | ||
* [[Hominy]] is also known in some areas of the Southeastern United States, as the breakfast food [[grits]], dried and ground into a coarse powder. They are prepared by boiling in water, with the addition of butter and other ingredient to suit the tastes of the preparer. | * [[Hominy]] is also known in some areas of the Southeastern United States, as the breakfast food [[grits]], dried and ground into a coarse powder. They are prepared by boiling in water, with the addition of butter and other ingredient to suit the tastes of the preparer. | ||
| Line 179: | Line 178: | ||
* German pretzels are poached in a boiling sodium hydroxide solution before baking, which contributes to their unique crust. | * German pretzels are poached in a boiling sodium hydroxide solution before baking, which contributes to their unique crust. | ||
| − | === Delignification of | + | === Delignification of cellulosic materials === |
| − | |||
Sodium Hydroxide, in addition to Sodium Sulfide, is a key component of the white liquor solution used to separate lignin from cellulose fibers in the [[Kraft process]]. It also plays a key role in several following stages of the process of bleaching the brown pulp resulting from the pulping process. These stages include oxygen delignification, oxidative extraction, and simple extraction, all of which require a strong alkaline environment with a pH > 10.5 at the end of the stages. | Sodium Hydroxide, in addition to Sodium Sulfide, is a key component of the white liquor solution used to separate lignin from cellulose fibers in the [[Kraft process]]. It also plays a key role in several following stages of the process of bleaching the brown pulp resulting from the pulping process. These stages include oxygen delignification, oxidative extraction, and simple extraction, all of which require a strong alkaline environment with a pH > 10.5 at the end of the stages. | ||
=== Domestic uses === | === Domestic uses === | ||
| − | Sodium hydroxide is used in the home as an agent for unblocking drains, provided as a dry crystal ( | + | Sodium hydroxide is used in the home as an agent for unblocking drains, provided as a dry crystal (for example, "[[Drāno]]") or as a thick liquid gel. The chemical mechanism employed is the conversion of grease to a form of [[soap]], and so forming a water soluble form to be dissolved by flushing; also decomposing complex molecules such as the [[protein]] of [[hair]]. Such '''drain cleaners''' (and their [[acid|acidic]] versions) are highly caustic and should be handled with care. |
| − | Beginning in the early 1900s, lye has been used to [[Relaxer|relax]] or straighten the hair of persons of African ethnicity. Among men, this treatment was often called a process. | + | Beginning in the early 1900s, lye has been used to [[Relaxer|relax]] or straighten the hair of persons of African ethnicity. Among men, this treatment was often called a process. However, because of the high incidence and intensity of chemical burns, chemical relaxer manufacturers began switching to other alkaline chemicals (most commonly [[guanidine hydroxide]]) during the latter quarter of the twentieth century, although lye relaxers are still available, usually under use by professionals. |
| − | === Tissue | + | === Tissue digestion === |
| − | This is a process that was used with farm animals at one time. This process involves the placing of a carcass into a sealed chamber, which then puts the carcass in a mixture of lye and water, which breaks chemical bonds keeping the body intact. This eventually turns the body into a coffee-like liquid, and the only solid remains are bone hulls, which could be crushed between one's fingertips | + | This is a process that was used with farm animals at one time. This process involves the placing of a carcass into a sealed chamber, which then puts the carcass in a mixture of lye and water, which breaks chemical bonds keeping the body intact. This eventually turns the body into a coffee-like liquid, and the only solid remains are bone hulls, which could be crushed between one's fingertips. |
| − | |||
| − | |||
=== Illegal drugs === | === Illegal drugs === | ||
| Line 199: | Line 195: | ||
Solid sodium hydroxide or solutions containing high concentrations of sodium hydroxide may cause [[chemical burn]]s, permanent injury or scarring, and [[blindness]]. | Solid sodium hydroxide or solutions containing high concentrations of sodium hydroxide may cause [[chemical burn]]s, permanent injury or scarring, and [[blindness]]. | ||
| − | Solvation of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables. | + | Solvation of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables. |
| − | The combination of aluminium and sodium hydroxide results in a large production of hydrogen gas:<br> | + | The combination of aluminium and sodium hydroxide results in a large production of hydrogen gas:<br/> |
| − | 2[[Aluminium|Al]]([[Solid|s]]) + 6NaOH(aq) → 3[[Hydrogen|H<sub>2</sub>]]([[Gas|g]]) + 2Na<sub>3</sub>AlO<sub>3</sub>(aq). <br> | + | 2[[Aluminium|Al]]([[Solid|s]]) + 6NaOH(aq) → 3[[Hydrogen|H<sub>2</sub>]]([[Gas|g]]) + 2Na<sub>3</sub>AlO<sub>3</sub>(aq). <br/> |
Mixing these two in a closed container is therefore dangerous. | Mixing these two in a closed container is therefore dangerous. | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
==Notes== | ==Notes== | ||
| − | |||
<references /> | <references /> | ||
| − | |||
| − | == | + | == References == |
| + | * Heaton, Alan, ed. 1996. ''An Introduction to Industrial Chemistry.'' Glasgow, UK: Blackie. ISBN 0-7514-0272-9 | ||
| + | * Moore, John T., Ed.D. 2004. ''Chemistry Made Simple''. New York: Broadway Books. ISBN 0767917022 | ||
| + | * Seidel, Arza, ed. 2006. ''Kirk-Othmer Encyclopedia of Chemical Technology''. Hoboken, NJ: John Wiley. ISBN 047148508X | ||
| + | |||
| + | == External links == | ||
| + | All links retrieved January 30, 2023. | ||
| − | * | + | * [http://www.cdc.gov/niosh/npg/npgd0565.html Sodium hydroxide.] ''NIOSH Pocket Guide to Chemical Hazards''. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Latest revision as of 15:05, 27 April 2023
| Sodium hydroxide | |
|---|---|
| |
| General | |
| Systematic name | Sodium hydroxide |
| Other names | Lye, Caustic Soda |
| Molecular formula | NaOH |
| Molar mass | 39.9971 g/mol |
| Appearance | White solid |
| CAS number | [1310-73-2] |
| Properties | |
| Density and phase | 2.1 g/cm³, solid |
| Solubility in water | 111 g/100 ml (20°C) |
| Melting point | 318°C (591 K) |
| Boiling point | 1390°C (1663 K) |
| Basicity (pKb) | -2.43 |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Corrosive (C) |
| R-phrases | R35 |
| S-phrases | S1/2, S26, S37/39, S45 |
| NFPA 704 | |
| Flash point | Non-flammable. |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | Sodium chloride Sodium sulfate. |
| Other cations | Potassium hydroxide Calcium hydroxide |
| Related bases | Ammonia, lime. |
| Related compounds | |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
Sodium hydroxide, also known as lye or caustic soda, is a caustic metallic base. Its chemical formula is NaOH. Forming a strongly alkaline solution when dissolved in a solvent such as water, caustic soda is widely used in many industries, mostly as a strong chemical base in the manufacture of pulp and paper, textiles, drinking water, soaps, and detergents. Worldwide production in 1998, was around 45 million tons. Sodium hydroxide is also the most common base used in chemical laboratories, and it is widely used as a drain cleaner.
General properties
Pure sodium hydroxide is a white solid; available in pellets, flakes, granules, and also as a 50-percent saturated solution. It is deliquescent and also readily absorbs carbon dioxide from the air, so it should be stored in an airtight container. It is very soluble in water, with liberation of heat. It also dissolves in ethanol and methanol, though it exhibits lower solubility in these solvents than does potassium hydroxide. It is insoluble in ether and other non-polar solvents. A sodium hydroxide solution will leave a yellow stain on fabric and paper.
Chemical properties
Sodium hydroxide is completely ionic, containing sodium ions and hydroxide ions. The hydroxide ion makes sodium hydroxide a strong base which reacts with acids to form water and the corresponding salts, for example, with hydrochloric acid, sodium chloride is formed:
In general, such neutralization reactions are represented by one simple net ionic equation:
This type of reaction releases heat when a strong acid is used. Such acid-base reactions can also be used for titrations, and indeed this is a common way for measuring the concentration of acids.
Related to this is the reaction of sodium hydroxide with acidic oxides. The reaction of carbon dioxide has already been mentioned, but other acidic oxides such as sulfur dioxide (SO2) also react completely. Such reactions are often used to "scrub" harmful acidic gases (like SO2 and H2S) and prevent their release into the atmosphere.
Sodium hydroxide slowly reacts with glass to form sodium silicate, so glass joints and stopcocks exposed to NaOH have a tendency to "freeze." Flasks and glass-lined chemical reactors are damaged by long exposure to hot sodium hydroxide, and the glass becomes frosted. Sodium hydroxide does not attack iron or copper, but many other metals such as aluminium, zinc, and titanium are attacked rapidly. In 1986, an aluminum road tanker in the UK was mistakenly used to transport 25 percent sodium hydroxide solution, causing pressurization of the contents and damage to the tanker. For this same reason aluminum pans should never be cleaned with lye.
Many non-metals also react with sodium hydroxide, giving salts. For example, phosphorus forms sodium hypophosphite, while silicon gives sodium silicate.
Unlike NaOH, the hydroxides of most metals are insoluble, and therefore sodium hydroxide can be used to precipitate metal hydroxides. One such hydroxide is aluminium hydroxide, used as a gelatinous floc to filter out particulate matter in water treatment. Aluminum hydroxide is prepared at the treatment plant from aluminum sulfate by reaction with NaOH:
Sodium hydroxide reacts readily with carboxylic acids to form their salts, and it is even a strong enough base to form salts with phenols. NaOH can also be used for the base-driven hydrolysis of esters (as is saponification), amides and alkyl halides. However, the limited solubility of NaOH in organic solvents means that the more soluble KOH is often preferred.
Manufacture
In 1998, total world production was around 45 million tons. Of this, both North America and Asia contributed around 14 million metric tons, and Europe produced around 10 million metric tons.
Methods of production
Sodium hydroxide is produced (along with chlorine and hydrogen) via the chloralkali process. This involves the electrolysis of an aqueous solution of sodium chloride. The sodium hydroxide builds up at the cathode, where water is reduced to hydrogen gas and hydroxide ion:
- 2Na+ + 2H2O + 2e− → H2 + 2NaOH
To produce NaOH, it is necessary to prevent reaction of the NaOH with the chlorine. This is typically done in one of three ways, of which the membrane cell process is economically the most viable.
- Mercury cell process (also called the Castner-Kellner process)—sodium metal forms as an amalgam at a mercury cathode; this sodium is then reacted with water to produce NaOH. There have been concerns about mercury releases, although modern plants claim to be safe in this regard.[1]
- Diaphragm cell process—uses a steel cathode, and reaction of NaOH with Cl2 is prevented using a porous diaphragm. In the diaphragm cell process, the anode area is separated from the cathode area by a permeable diaphragm. The brine is introduced into the anode compartment and flows through the diaphragm into the cathode compartment. A diluted caustic brine leaves the cell. The caustic soda must usually be concentrated to 50 percent and the salt removed. This is done using an evaporative process with about three metric tons of steam per metric ton of caustic soda. The salt separated from the caustic brine can be used to saturate diluted brine. The chlorine contains oxygen and must often be purified by liquefaction and evaporation.[2]
- Membrane cell process—similar to the diaphragm cell process, with a Nafion membrane to separate the cathode and anode reactions. Only sodium ions and a little water pass through the membrane. It produces a higher quality of NaOH. Of the three processes, the membrane cell process requires the lowest consumption of electric energy and the amount of steam needed for concentration of the caustic is relatively small (less than one metric ton per metric ton of caustic soda).[3]
An older method for sodium hydroxide production was the LeBlanc process, which produced sodium carbonate, followed by roasting, to create carbon dioxide and sodium oxide. This method is still occasionally used. It helped to establish sodium hydroxide as an important commodity chemical.
Major producers
In the United States, the major producer of sodium hydroxide is the Dow Chemical Company, which has annual production around 3.7 million tons from sites at Freeport, Texas, and Plaquemine, Louisiana. Other major U.S. producers include Oxychem, PPG, Olin, Pioneer Companies, Inc. (PIONA), and Formosa. All of these companies use the chloralkali process.[4]
Uses
General applications
Sodium hydroxide is the principal strong base used in the chemical industry. In bulk, it is most often handled as an aqueous solution, since solutions are cheaper and easier to handle. It is used to drive for chemical reactions and also for the neutralization of acidic materials. It can be used also as a neutralizing agent in petroleum refining.
Gold pennies
Sodium hydroxide has also been used in conjunction with zinc for creation of the famous "Gold pennies" experiment. When a penny is boiled in a solution of NaOH together with some granular zinc metal (galvanized nails are one source), the color of the penny will turn silver in about 45 seconds. The penny is then held in the flame of a burner for a few seconds and it turns golden. The reason this happens is that granular zinc dissolves in NaOH to form Zn(OH)42-. This zincate ion becomes reduced to metallic zinc on the surface of a copper penny. Zinc and copper when heated in a flame form brass.
Use in chemical analysis
In analytical chemistry, sodium hydroxide solutions are often used to measure the concentration of acids by titration. Since NaOH is not a primary standard, solutions must first be standardized by titration against a standard such as KHP. Burettes exposed to NaOH should be rinsed out immediately after use to prevent "freezing" of the stopcock. Sodium hydroxide was traditionally used to test for cations in Qualitative Inorganic Analysis, as well as to provide alkaline media for some reactions that need it, such as the Biuret test.
Soap making
Soap making (cold process soap, saponification) is the most traditional chemical process using sodium hydroxide. The Arabs began producing soap in this way in the seventh century, and the same basic process is still used today.
Biodiesel
For the manufacture of biodiesel, sodium hydroxide is used as a catalyst for the transesterification of methanol and triglycerides. This only works with anhydrous sodium hydroxide, because water and lye would turn the fat into soap which would be tainted with methanol.
It is used more often than potassium hydroxide because it costs less, and a smaller quantity is needed for the same results. Another alternative is sodium silicate.
Aluminum etching
Strong bases attack aluminum. This can be useful in etching through a resist or in converting a polished surface to a satin-like finish, but without further passivation such as anodizing or allodizing the surface may become corroded, either under normal use or in severe atmospheric conditions.
Food preparation
Food uses of lye include washing or chemical peeling of fruits and vegetables, chocolate and cocoa processing, caramel color production, poultry scalding, soft drink processing, and thickening ice cream. Olives are often soaked in lye to soften them, while pretzels and German lye rolls are glazed with a lye solution before baking to make them crisp.
Specific foods processed with lye include:
- The Scandinavian delicacy known as lutefisk (from lutfisk, "lye fish").
- Hominy is dried maize (corn) kernels reconstituted by soaking in lye-water. These expand considerably in size and may be further processed by cooking in hot oil and salting to form corn nuts. Nixtamal is similar, but uses calcium hydroxide instead of sodium hydroxide.
- Hominy is also known in some areas of the Southeastern United States, as the breakfast food grits, dried and ground into a coarse powder. They are prepared by boiling in water, with the addition of butter and other ingredient to suit the tastes of the preparer.
- Sodium hydroxide is also the chemical that causes gelling of egg whites in the production of Century eggs.
- German pretzels are poached in a boiling sodium hydroxide solution before baking, which contributes to their unique crust.
Delignification of cellulosic materials
Sodium Hydroxide, in addition to Sodium Sulfide, is a key component of the white liquor solution used to separate lignin from cellulose fibers in the Kraft process. It also plays a key role in several following stages of the process of bleaching the brown pulp resulting from the pulping process. These stages include oxygen delignification, oxidative extraction, and simple extraction, all of which require a strong alkaline environment with a pH > 10.5 at the end of the stages.
Domestic uses
Sodium hydroxide is used in the home as an agent for unblocking drains, provided as a dry crystal (for example, "Drāno") or as a thick liquid gel. The chemical mechanism employed is the conversion of grease to a form of soap, and so forming a water soluble form to be dissolved by flushing; also decomposing complex molecules such as the protein of hair. Such drain cleaners (and their acidic versions) are highly caustic and should be handled with care.
Beginning in the early 1900s, lye has been used to relax or straighten the hair of persons of African ethnicity. Among men, this treatment was often called a process. However, because of the high incidence and intensity of chemical burns, chemical relaxer manufacturers began switching to other alkaline chemicals (most commonly guanidine hydroxide) during the latter quarter of the twentieth century, although lye relaxers are still available, usually under use by professionals.
Tissue digestion
This is a process that was used with farm animals at one time. This process involves the placing of a carcass into a sealed chamber, which then puts the carcass in a mixture of lye and water, which breaks chemical bonds keeping the body intact. This eventually turns the body into a coffee-like liquid, and the only solid remains are bone hulls, which could be crushed between one's fingertips.
Illegal drugs
Sodium hydroxide is a key reagent in the process of making Methamphetamine and other illegal drugs. Contrary to popular media reports, it is not actually an "ingredient" in these drugs, but simply a strong base used to manipulate the pH at various points in a chemical synthesis.
Safety
Solid sodium hydroxide or solutions containing high concentrations of sodium hydroxide may cause chemical burns, permanent injury or scarring, and blindness.
Solvation of sodium hydroxide is highly exothermic, and the resulting heat may cause heat burns or ignite flammables.
The combination of aluminium and sodium hydroxide results in a large production of hydrogen gas:
2Al(s) + 6NaOH(aq) → 3H2(g) + 2Na3AlO3(aq).
Mixing these two in a closed container is therefore dangerous.
Notes
- ↑ Euro Chlor, Chlorine Online Diagram of mercury cell process. Retrieved April 4, 2008.
- ↑ Euro Chlor, How is chlorine made? Retrieved April 4, 2008.
- ↑ Euro Chlor, Chlorine Online Diagram of membrane cell process. Retrieved April 4, 2008.
- ↑ Kirk-Othmer Encyclopedia of Chemical Technology.
ReferencesISBN links support NWE through referral fees
- Heaton, Alan, ed. 1996. An Introduction to Industrial Chemistry. Glasgow, UK: Blackie. ISBN 0-7514-0272-9
- Moore, John T., Ed.D. 2004. Chemistry Made Simple. New York: Broadway Books. ISBN 0767917022
- Seidel, Arza, ed. 2006. Kirk-Othmer Encyclopedia of Chemical Technology. Hoboken, NJ: John Wiley. ISBN 047148508X
External links
All links retrieved January 30, 2023.
- Sodium hydroxide. NIOSH Pocket Guide to Chemical Hazards.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.