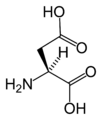
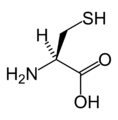
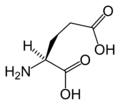
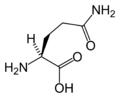
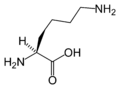
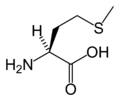
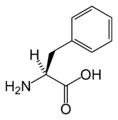
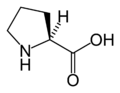
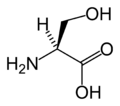
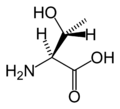
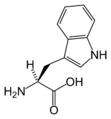
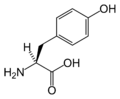
Amino acid
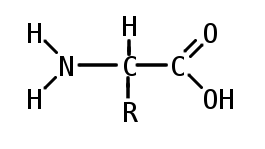
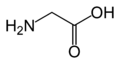
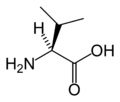
An amino acid is an organic molecule with three main components: an amino group (-NH2), a carboxylic acid group (-COOH), and an R group (or side chain) unique to each amino acid.
Amino acids are the basic structural building blocks of proteins. Just as the letters of the alphabet can be combined in different ways to form an endless variety of words, a limited number of amino acids can be linked together in varying sequences to form a vast array of proteins. The unique three-dimensional shape of each protein, which results from the linear sequence of amino acids, determines the protein's specific function in the body.
Although over 100 amino acids exist in nature, our bodies require about 20 amino acids, called standard amino acids, for normal functioning. Nine of these standard amino acids are considered essential—that is, the body cannot synthesize them by itself and must obtain them from food.
Sources of amino acids
Over one hundred amino acids have been found in nature. Some of these have been detected in meteorites, especially in a type known as carbonaceous chondrites (whose composition is considered to be representative of the solar nebula, or gaseous cloud, from which the solar system condensed). Microorganisms and plants can produce uncommon amino acids, which can be found in peptidic antibiotics such as nisin, which is used as a food preservative.
Standard amino acids
Twenty amino acids are known as standard amino acids or proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acids are found in proteins and are encoded by the standard genetic code. They are formed from an mRNA template in a process called translation, which is part of protein synthesis. Combinations of these amino acids produce every single essential protein for the homeostasis (or maintenance of a stable internal environment) of the human body.
L-Alanine (Ala / A)
L-Arginine (Arg / R)
L-Asparagine (Asn / N)
L-Aspartic acid (Asp / D)
L-Cysteine (Cys / C)
L-Glutamic Acid (Glu / E)
L-Glutamine (Gln / Q)
L-Glycine (Gly / G)
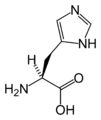
L-Histidine (His / H)
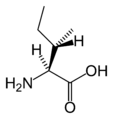
L-Isoleucine (Ile / I)
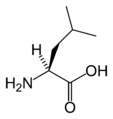
L-Leucine (Leu / L)
L-Lysine (Lys / K)
L-Methionine (Met / M)
L-Phenylalanine (Phe / F)
L-Proline (Pro / P)
L-Serine (Ser / S)
L-Threonine (Thr / T)
L-Tryptophan (Trp / W)
L-Tyrosine (Tyr / Y)
L-Valine (Val / V)
Chemical structures of the 20 standard amino acids.
Essential amino acids
Nine of the 20 standard amino acids are called essential amino acids because they cannot be synthesized by the body from other compounds through chemical reactions, but instead must be taken in with food. In humans, the essential amino acids are lysine, histidine, isoleucine, phenylalanine, leucine, methionine, tryptophan, threonine and valine. Arginine and histidine are generally considered essential only in infants, because of their inability to synthesise them given their undeveloped metabolism?
The remaining 11 standard amino acids are nonessential; although they can be obtained from food, the body can also synthesize them as needed.
Nonstandard amino acids
Aside from the twenty standard amino acids and the two special amino acids, selenocysteine and pyrrolysine, which are coded by DNA in a non-standard manner, there are a vast number of "nonstandard amino acids". Non-proteinogenic amino acids are either not found in proteins (like carnitine, GABA, or L-DOPA), or not coded for in the standard genetic code (like hydroxyproline and selenomethionine). The latter often result from posttranslational modification of proteins; that is, the modification of standard amino acids after the protein has been formed in the translation stage of protein synthesis.
The structure of amino acids
In biochemistry, the term "amino acid" is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon. The general structure of proteinogenic alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid. The exception is proline, whose side chain cyclizes onto the backbone. This ring structure prevents proline from having a primary amino group; it is replaced by a secondary amino group.
When amino acids join to form proteins, the basic amino group and acidic carboxyl group are neutralized as they form a peptide chain. Instead, it is the side chains of the amino acids determine the acid-base properties of proteins. Thus, amino acids are usually classified by the properties of the side chain by two main characteristics: the charge of the R group (which determines whether the protein acts like a weak acid or a weak base), and their polarity (or tendency to interact with water at a neutral pH).
These properties influence the amino acids' interaction with other structures, both within the protein itself and among proteins. For example, soluble proteins have surfaces rich with polar amino acids like serine and threonine, while integral membrane proteins tend to have outer ring of hydrophobic amino acids that anchors them to the lipid bilayer, and proteins anchored to the membrane have a hydrophobic end that locks into the membrane. Similarly, proteins that have to bind to positively-charged molecules have surfaces rich with negatively charged amino acids like glutamate and aspartate, while proteins binding to negatively-charged molecules have surfaces rich with positively charged chains like lysine and arginine.
Isomers
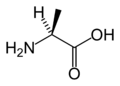
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. D amino acids are found in some proteins produced by exotic sea-dwelling organisms, such as cone snails. They are also abundant components of the proteoglycan cell walls of bacteria.
Amino acids function in protein synthesis
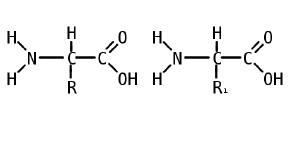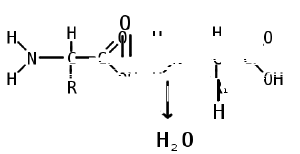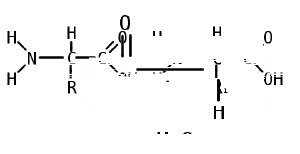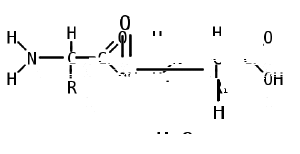
Two amino acids are linked together by a peptide bond, which formed when the basic amino group of one amino acid reacts with the acidic carboyxl group of a second amino acid. This condensation reaction (water loss) yields a peptide bond and a molecule of water. An amino acid residue is what is left of an amino acid once a molecule of water has been lost (an H+ from the amino group side and an OH- from the carboxylic side) in the formation of a peptide bond.
Proteins are then created by the polymerization of amino acids, a process in which amino acids are joined together in chains called, depending on their length, peptides or polypeptides.
Other biological roles of amino acids
In addition to protein synthesis, amino acids have other biologically-important roles. Glycine and glutamate are neurotransmitters as well as standard amino acids in proteins. Many amino acids are used to synthesize other molecules, for example:
- tryptophan is a precursor of the neurotransmitter serotonin
- glycine is one of the reactants in the synthesis of porphyrins such as heme
- arginine is used to synthesize the hormone nitric oxide
Numerous non-standard amino acids are also biologically-important: Gamma-aminobutyric acid is another neurotransmitter, carnitine is used in lipid transport within a cell, ornithine, citrulline, homocysteine, hydroxyproline, hydroxylysine, and sarcosine.
- Aspartame (aspartyl-phenylalanine-1-methyl ester) is an artificial sweetener.
- 5-HTP (5-hydroxytryptophan) has been used to treat neurological problems associated with PKU (phenylketonuria), as well as depression (as an alternative to L-Tryptophan).
- L-DOPA (L-dihydroxyphenylalanine) is a drug used to treat Parkinsonism.
- Monosodium glutamate is a food additive to enhance flavor.
Table of chemical properties
Following is a table listing the one letter symbols, the three-letter symbols, and the chemical properties of the side chains of the standard amino acids. The mass listed is the weighted average of all common isotopes, and includes the mass of H2O. The one-letter symbol for an undetermined amino acid is X. The three-letter symbol Asx or one-letter symbol B means the amino acid is either asparagine or aspartic acid, whereas Glx or Z means either glutamic acid or glutamine. The three-letter symbol Sec or one-letter symbol U refers to selenocysteine. The letters J and O are not used.
| Abbrev. | Full Name | Side chain type | Mass | pI | pK1 (α-COOH) |
pK2 (α-+NH3) |
pKr (R) | Remarks | |
|---|---|---|---|---|---|---|---|---|---|
| A | Ala | Alanine | hydrophobic | 89.09 | 6.01 | 2.35 | 9.87 | Very abundant, very versatile. More stiff than glycine, but small enough to pose only small steric limits for the protein conformation. It behaves fairly neutrally, can be located in both hydrophilic regions on the protein outside and the hydrophobic areas inside. | |
| C | Cys | Cysteine | hydrophobic (Nagano, 1999) | 121.16 | 5.05 | 1.92 | 10.70 | 8.18 | The sulfur atom binds readily to heavy metal ions. Under oxidizing conditions, two cysteines can join together by a disulfide bond to form the amino acid cystine. When cystines are part of a protein, insulin for example, this enforces tertiary structure and makes the protein more resistant to unfolding and denaturation; disulphide bridges are therefore common in proteins that have to function in harsh environments, digestive enzymes (e.g., pepsin and chymotrypsin), structural proteins (e.g., keratin), and proteins too small to hold their shape on their own (eg. insulin). |
| D | Asp | Aspartic acid | acidic | 133.10 | 2.85 | 1.99 | 9.90 | 3.90 | Behaves similarly to glutamic acid. Carries a hydrophilic acidic group with strong negative charge. Usually is located on the outer surface of the protein, making it water-soluble. Binds to positively-charged molecules and ions, often used in enzymes to fix the metal ion. When located inside of the protein, aspartate and glutamate are usually paired with arginine and lysine. |
| E | Glu | Glutamic acid | acidic | 147.13 | 3.15 | 2.10 | 9.47 | 4.07 | Behaves similar to aspartic acid. Has longer, slightly more flexible side chain. |
| F | Phe | Phenylalanine | hydrophobic | 165.19 | 5.49 | 2.20 | 9.31 | Essential for humans. Phenylalanine, tyrosine, and tryptophan contain large rigid aromatic group on the side chain. These are the biggest amino acids. Like isoleucine, leucine and valine, these are hydrophobic and tend to orient towards the interior of the folded protein molecule. | |
| G | Gly | Glycine | hydrophobic | 75.07 | 6.06 | 2.35 | 9.78 | Because of the two hydrogen atoms at the α carbon, glycine is not optically active. It is the smallest amino acid, rotates easily, adds flexibility to the protein chain. It is able to fit into the tightest spaces, e.g., the triple helix of collagen. As too much flexibility is usually not desired, as a structural component it is less common than alanine. | |
| H | His | Histidine | basic | 155.16 | 7.60 | 1.80 | 9.33 | 6.04 | In even slightly acidic conditions protonation of the nitrogen occurs, changing the properties of histidine and the polypeptide as a whole. It is used by many proteins as a regulatory mechanism, changing the conformation and behavior of the polypeptide in acidic regions such as the late endosome or lysosome, enforcing conformation change in enzymes. However only a few histidines are needed for this, so it is comparatively scarce. |
| I | Ile | Isoleucine | hydrophobic | 131.17 | 6.05 | 2.32 | 9.76 | Essential for humans. Isoleucine, leucine and valine have large aliphatic hydrophobic side chains. Their molecules are rigid, and their mutual hydrophobic interactions are important for the correct folding of proteins, as these chains tend to be located inside of the protein molecule. | |
| K | Lys | Lysine | basic | 146.19 | 9.60 | 2.16 | 9.06 | 10.54 | Essential for humans. Behaves similarly to arginine. Contains a long flexible side-chain with a positively-charged end. The flexibility of the chain makes lysine and arginine suitable for binding to molecules with many negative charges on their surfaces. E.g., DNA-binding proteins have their active regions rich with arginine and lysine. The strong charge makes these two amino acids prone to be located on the outer hydrophilic surfaces of the proteins; when they are found inside, they are usually paired with a corresponding negatively-charged amino acid, e.g., aspartate or glutamate. |
| L | Leu | Leucine | hydrophobic | 131.17 | 6.01 | 2.33 | 9.74 | Essential for humans. Behaves similar to isoleucine and valine. See isoleucine. | |
| M | Met | Methionine | hydrophobic | 149.21 | 5.74 | 2.13 | 9.28 | Essential for humans. Always the first amino acid to be incorporated into a protein; sometimes removed after translation. Like cysteine, contains sulfur, but with a methyl group instead of hydrogen. This methyl group can be activated, and is used in many reactions where a new carbon atom is being added to another molecule. | |
| N | Asn | Asparagine | hydrophilic | 132.12 | 5.41 | 2.14 | 8.72 | Neutralized version of aspartic acid. | |
| P | Pro | Proline | hydrophobic | 115.13 | 6.30 | 1.95 | 10.64 | Contains an unusual ring to the N-end amine group, which forces the CO-NH amide sequence into a fixed conformation. Can disrupt protein folding structures like α helix or β sheet, forcing the desired kink in the protein chain. Common in collagen, where it undergoes a posttranslational modification to hydroxyproline. Uncommon elsewhere. | |
| Q | Gln | Glutamine | hydrophilic | 146.15 | 5.65 | 2.17 | 9.13 | Neutralized version of glutamic acid. Used in proteins and as a storage for ammonia. | |
| R | Arg | Arginine | basic | 174.20 | 10.76 | 1.82 | 8.99 | 12.48 | Functionally similar to lysine. |
| S | Ser | Serine | hydrophilic | 105.09 | 5.68 | 2.19 | 9.21 | Serine and threonine have a short group ended with a hydroxyl group. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in enzymes. Both are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them. | |
| T | Thr | Threonine | hydrophilic | 119.12 | 5.60 | 2.09 | 9.10 | Essential for humans. Behaves similarly to serine. | |
| V | Val | Valine | hydrophobic | 117.15 | 6.00 | 2.39 | 9.74 | Essential for humans. Behaves similarly to isoleucine and leucine. See isoleucine. | |
| W | Trp | Tryptophan | hydrophobic | 204.23 | 5.89 | 2.46 | 9.41 | Essential for humans. Behaves similarly to phenylalanine and tyrosine (see phenylalanine). Precursor of serotonin. | |
| Y | Tyr | Tyrosine | hydrophobic | 181.19 | 5.64 | 2.20 | 9.21 | 10.46 | Behaves similarly to phenylalanine and tryptophan (see phenylalanine). Precursor of melanin, epinephrine, and thyroid hormones. |
| Amino acid | Abbrev. | Side chain | Hydro- phobic | Polar | Charged | Small | Tiny | Aromatic or Aliphatic | van der Waals volume | Codon | Occurrence in proteins (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Alanine | Ala, A | -CH3 | X | - | - | X | X | - | 67 | GCU, GCC, GCA, GCG | 7.8 |
| Cysteine | Cys, C | -CH2SH | X | - | - | X | - | - | 86 | UGU, UGC | 1.9 |
| Aspartate | Asp, D | -CH2COOH | - | X | negative | X | - | - | 91 | GAU, GAC | 5.3 |
| Glutamate | Glu, E | -CH2CH2COOH | - | X | negative | - | - | - | 109 | GAA, GAG | 6.3 |
| Phenylalanine | Phe, F | -CH2C6H5 | X | - | - | - | - | Aromatic | 135 | UUU, UUC | 3.9 |
| Glycine | Gly, G | -H | X | - | - | X | X | - | 48 | GGU, GGC, GGA, GGG | 7.2 |
| Histidine | His, H | -CH2-C3H3N2 | - | X | positive | - | - | Aromatic | 118 | CAU, CAC | 2.3 |
| Isoleucine | Ile, I | -CH(CH3)CH2CH3 | X | - | - | - | - | Aliphatic | 124 | AUU, AUC, AUA | 5.3 |
| Lysine | Lys, K | -(CH2)4NH2 | - | X | positive | - | - | - | 135 | AAA, AAG | 5.9 |
| Leucine | Leu, L | -CH2CH(CH3)2 | X | - | - | - | - | Aliphatic | 124 | UUA, UUG, CUU, CUC, CUA, CUG | 9.1 |
| Methionine | Met, M | -CH2CH2SCH3 | X | - | - | - | - | - | 124 | AUG | 2.3 |
| Asparagine | Asn, N | -CH2CONH2 | - | X | - | X | - | - | 96 | AAU, AAC | 4.3 |
| Proline | Pro, P | -CH2CH2CH2- | X | - | - | X | - | - | 90 | CCU, CCC, CCA, CCG | 5.2 |
| Glutamine | Gln, Q | -CH2CH2CONH2 | - | X | - | - | - | - | 114 | CAA, CAG | 4.2 |
| Arginine | Arg, R | -(CH2)3NH-C(NH)NH2 | - | X | positive | - | - | - | 148 | CGU, CGC, CGA, CGG, AGA, AGG | 5.1 |
| Serine | Ser, S | -CH2OH | - | X | - | X | X | - | 73 | UCU, UCC, UCA, UCG, AGU,AGC | 6.8 |
| Threonine | Thr, T | -CH(OH)CH3 | X | X | - | X | - | - | 93 | ACU, ACC, ACA, ACG | 5.9 |
| Valine | Val, V | -CH(CH3)2 | X | - | - | X | - | Aliphatic | 105 | GUU, GUC, GUA, GUG | 6.6 |
| Tryptophan | Trp, W | -CH2C8H6N | X | - | - | - | - | Aromatic | 163 | UGG | 1.4 |
| Tyrosine | Tyr, Y | -CH2-C6H4OH | X | X | - | - | - | Aromatic | 141 | UAU, UAC | 3.2 |
Note: The pKa values of amino acids are typically slightly different when the amino acid is inside a protein. Protein pKa calculations are sometimes used to calculate the change in the pKa value of an amino acid in this situation
ReferencesISBN links support NWE through referral fees
- Doolittle, R.F. (1989) Redundancies in protein sequences. In Predictions of Protein Structure and the Principles of Protein Conformation (Fasman, G.D. ed) Plenum Press, New York, pp. 599-623
- David L. Nelson and Michael M. Cox, Lehninger Principles of Biochemistry, 3rd edition, 2000, Worth Publishers, ISBN 1572591536
- On the hydrophobic nature of cysteine.
External links
- Molecular Expressions: The Amino Acid Collection - Has detailed information and microscopy photographs of each amino acid.
- 22nd amino acid - Press release from Ohio State claiming discovery of a 22nd amino acid.
- Amino acid properties - Properties of the amino acids (a tool aimed mostly and molecular geneticists trying to understand the meaning of mutations)
- Synthesis of Amino Acids and Derivatives
- Right-handed amino acids were left behind
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.