Difference between revisions of "Transition metal" - New World Encyclopedia
(→Electronic configuration: editing) |
(→Properties: editing) |
||
| Line 77: | Line 77: | ||
There are several common characteristic properties of transition elements: | There are several common characteristic properties of transition elements: | ||
| − | *Almost all of them are solids at room temperature, with high [[tensile strength]], [[density]], and [[melting point|melting]] and [[boiling point|boiling]] points. The one exception is [[mercury (element)|mercury]], which is a liquid. | + | *Almost all of them are solids at room temperature, with high [[tensile strength]] (ability to withstand stress), [[density]], and [[melting point|melting]] and [[boiling point|boiling]] points. The one exception is [[mercury (element)|mercury]], which is a liquid. |
*Most of them are silvery-blue at room temperature. The exceptions are [[copper]] and [[gold]]. | *Most of them are silvery-blue at room temperature. The exceptions are [[copper]] and [[gold]]. | ||
*They form monatomic ions with a 2+ charge, but can form other ions with a different charge. For example, iron can form Fe<sup>2+</sup> and Fe<sup>3+</sup> ions. In addition, they often have higher [[oxidation state]]s in compounds. | *They form monatomic ions with a 2+ charge, but can form other ions with a different charge. For example, iron can form Fe<sup>2+</sup> and Fe<sup>3+</sup> ions. In addition, they often have higher [[oxidation state]]s in compounds. | ||
*They form complexes known as "[[coordination compounds]]," many of which are brightly colored. | *They form complexes known as "[[coordination compounds]]," many of which are brightly colored. | ||
| − | *They are often good [[catalyst]]s. | + | *They are often good [[catalyst]]s. For example, [[iron]] is the catalyst for the [[Haber process]]*, involving the reaction of [[nitrogen]] and [[hydrogen]] to produce [[ammonia]]*. [[Nickel]], [[palladium]], or [[platinum]] can be used in the hydrogenation of (addition of hydrogen atoms to) [[alkenes]] and [[alkynes]]. Platinum is the catalyst in the [[catalytic converter]]s of automobile exhaust systems. |
In addition to these common characteristics, there are some trends in properties as we go through a period, much like those in the [[Periodic table, main group elements|main group elements]], but with less dramatic changes. Going across the transition metals of a period, the atomic radius generally tends to decrease, and the first [[ionization energy]] increases. Also, as we go across the period, the metals tend to become softer, and mercury is a liquid at room temperature. Group 11 elements (copper, silver, and gold) are particularly unreactive. These "noble" metals can occur naturally in their elemental metallic state, and they are sometimes known as coinage metals as they have been useful for minting coins. | In addition to these common characteristics, there are some trends in properties as we go through a period, much like those in the [[Periodic table, main group elements|main group elements]], but with less dramatic changes. Going across the transition metals of a period, the atomic radius generally tends to decrease, and the first [[ionization energy]] increases. Also, as we go across the period, the metals tend to become softer, and mercury is a liquid at room temperature. Group 11 elements (copper, silver, and gold) are particularly unreactive. These "noble" metals can occur naturally in their elemental metallic state, and they are sometimes known as coinage metals as they have been useful for minting coins. | ||
| Line 98: | Line 98: | ||
== Variable oxidation states == | == Variable oxidation states == | ||
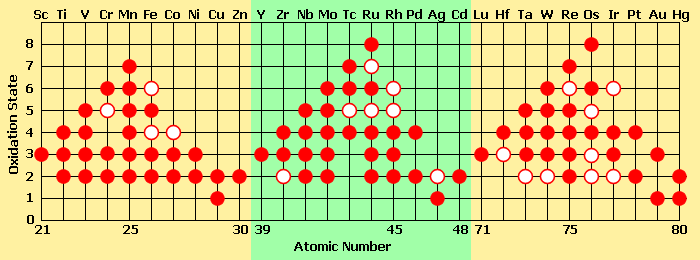
| − | Unlike ions of most [[main group elements|main group]] metals monatomic ions of the transition metals may have more than one stable charge, and in compounds several higher [[oxidation states]]. This is because they can lose ''d'' electrons without a high energetic penalty. | + | Unlike ions of most [[main group elements|main group]] metals, monatomic ions of the transition metals may have more than one stable charge, and, in compounds, they can have several higher [[oxidation states]]. This is because they can lose ''d'' electrons without a high energetic penalty. Manganese, for example, has two 4''s'' electrons and five 3''d'' electrons, which can be removed or shared with other atoms. Loss or sharing of all of these electrons leads to a 7+ oxidation state. [[Osmium]] and [[ruthenium]] compounds are commonly isolated in stable 8+ oxidation states, which is among the highest for isolable compounds. |
| − | [[Image:Transition metal oxidation states 2.png|center|frame|This table shows some of the oxidation states found in compounds of the transition | + | [[Image:Transition metal oxidation states 2.png|center|frame|This table shows some of the oxidation states found in compounds of the transition metals.<br> A solid circle represents a common oxidation state, and a ring represents a less common (energetically less favorable) oxidation state.]] |
| − | + | Moving across a period of transition elements, certain patterns in their oxidation states emerge: | |
| − | *The number of oxidation states of each ion increases up to | + | *The number of oxidation states of each ion increases up to manganese (group 7), after which they decrease. Later transition metals have a stronger attraction between [[proton]]s and [[electron]]s (because there are more of them present), requiring more energy to remove the electrons. |
| − | *When | + | *When these elements are in lower oxidation states, they can be found as simple ions. In their higher oxidation states, these elements are usually bonded covalently to electronegative elements like oxygen or fluorine, forming [[polyatomic ion]]s such as [[chromate]]*, [[vanadate]]*, or [[permanganate]]*. |
| − | Other properties with | + | Other properties associated with the stability of oxidation states are as follows: |
| − | *Ions in higher oxidation states tend to make good | + | *Ions in higher oxidation states tend to make good oxidizing agents, whereas elements in low oxidation states become reducing agents. |
| − | * | + | *Going across a period, the 2+ ions start as strong [[reducing agent]]s and increase in stability. |
| − | * | + | *Conversely, the 3+ ions start at higher stability and become more [[oxidizing agent|oxidizing]] across the period. |
== Colored compounds == | == Colored compounds == | ||
| Line 124: | Line 124: | ||
Interestingly, though [[zinc]] can form complexes they are colorless because the 3d orbitals of zinc are full. The full ''d'' orbitals prevent the complex from absorbing visible light when the energies of the ''d'' orbitals are altered by the ligands. Zinc is in group 12, so is not considered a transition metal in the newer IUPAC definition | Interestingly, though [[zinc]] can form complexes they are colorless because the 3d orbitals of zinc are full. The full ''d'' orbitals prevent the complex from absorbing visible light when the energies of the ''d'' orbitals are altered by the ligands. Zinc is in group 12, so is not considered a transition metal in the newer IUPAC definition | ||
| − | + | ||
| − | |||
==Reference== | ==Reference== | ||
Revision as of 15:23, 14 June 2006
The elements that divide the main groups of the periodic table in the standard view of the table, that is the elements in groups 3 through 12, are commonly called transition metals or transition elements. The name transition comes from their position in the table. They divide the periods of the table so are a transition between groups 2 and 13. Some of these elements occur naturally in their metallic state and have been known since antiquity. Three of these, gold, silver, and copper are important economically and have been extensively used in coinage and jewelry. Use of copper in tools was one of the first historical technological advances. Transition metals provide some of the important metallic catalysts used in industrial and laboratory settings, and iron in the form of steel is used in many things from cars to bridges.
| The Transition Metals | ||||||||||
| Group | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|
| Period 4 | Sc 21 | Ti 22 | V 23 | Cr 24 | Mn 25 | Fe 26 | Co 27 | Ni 28 | Cu 29 | Zn 30 |
| Period 5 | Y 39 | Zr 40 | Nb 41 | Mo 42 | Tc 43 | Ru 44 | Rh 45 | Pd 46 | Ag 47 | Cd 48 |
| Period 6 | Lu 71 | Hf 72 | Ta 73 | W 74 | Re 75 | Os 76 | Ir 77 | Pt 78 | Au 79 | Hg 80 |
| Period 7 | Lr 103 | Rf 104 | Db 105 | Sg 106 | Bh 107 | Hs 108 | Mt 109 | Ds 110 | Rg 111 | Uub 112 |
Definitions
The general definition of transition metals as those that lie in groups 3 through 12 (the "d-block" elements) of the periodic table, mentioned above, is simple and has been traditionally used. Although this definition is still widely used, the characteristic properties of transition metals arise because of the electron configuration of their atoms, which have partially filledd orbitals. This has led to a stricter definition of the term. The International Union of Pure and Applied Chemistry (IUPAC) defines a transition element as "an element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell" [1].
By this definition, zinc, cadmium, and mercury (group 12 elements) are not considered to be transition metals. This is because the atoms of these elements and their stable ions contain electrons that completely fill the d orbitals. When these elements form ions, they usually lose electrons from only their outermost s subshell, leaving the d subshell intact. In just a few, exceptional cases, they have formed unstable ions in which the d subshell is partly filled.[1] Element 112 (in group 12) may also be excluded, because its electron configuration is likely to be similar to that of other members of group 12, and its oxidation properties are unlikely to be observed due to its radioactive nature. Thus, this stricter definition of transition metals limits the term to elements in groups 3 to 11.
Properties
There are several common characteristic properties of transition elements:
- Almost all of them are solids at room temperature, with high tensile strength (ability to withstand stress), density, and melting and boiling points. The one exception is mercury, which is a liquid.
- Most of them are silvery-blue at room temperature. The exceptions are copper and gold.
- They form monatomic ions with a 2+ charge, but can form other ions with a different charge. For example, iron can form Fe2+ and Fe3+ ions. In addition, they often have higher oxidation states in compounds.
- They form complexes known as "coordination compounds," many of which are brightly colored.
- They are often good catalysts. For example, iron is the catalyst for the Haber process, involving the reaction of nitrogen and hydrogen to produce ammonia. Nickel, palladium, or platinum can be used in the hydrogenation of (addition of hydrogen atoms to) alkenes and alkynes. Platinum is the catalyst in the catalytic converters of automobile exhaust systems.
In addition to these common characteristics, there are some trends in properties as we go through a period, much like those in the main group elements, but with less dramatic changes. Going across the transition metals of a period, the atomic radius generally tends to decrease, and the first ionization energy increases. Also, as we go across the period, the metals tend to become softer, and mercury is a liquid at room temperature. Group 11 elements (copper, silver, and gold) are particularly unreactive. These "noble" metals can occur naturally in their elemental metallic state, and they are sometimes known as coinage metals as they have been useful for minting coins.
Electronic configuration
- Main article: electron configuration
The properties of transition metals arise from their defining characteristic of partially filled d orbitals. They are metals because the d orbital electrons are delocalized within the metal lattice, forming metallic bonds.
Most transition metals have two electrons in their outermost, s subshell. As we consider these elements across a period, the number of d electrons increases by one. Thus, in the fourth period, scandium (Sc, group 3) has the configuration [Ar]4s23d1, and the next element Titanium (Ti, group 4) has the configuration [Ar]4s23d2, and so forth. There are, however, some exceptions to this progression. For instance, in the fourth period, copper has the configuration ([Ar]4s13d10) and chromium is ([Ar]4s13d5). These exceptions occur because the atoms acquire additional stability when their subshells are half-filled or fully filled. Copper has a completely filled d subshell, and chromium has a half-filled d subshell. Similar exceptions are more prevalent in the fifth, sixth, and seventh periods.
When these metals lose electrons to form monatomic ions, they generally lose their s electrons first. Thus, most transition metals form ions with a 2+ charge. Higher oxidation states involve d electrons as well. Monatomic ions with a charge greater than 3+ are rare, and the higher oxidation states of transition metals occur in compounds with highly electronegative elements such as oxygen.
Variable oxidation states
Unlike ions of most main group metals, monatomic ions of the transition metals may have more than one stable charge, and, in compounds, they can have several higher oxidation states. This is because they can lose d electrons without a high energetic penalty. Manganese, for example, has two 4s electrons and five 3d electrons, which can be removed or shared with other atoms. Loss or sharing of all of these electrons leads to a 7+ oxidation state. Osmium and ruthenium compounds are commonly isolated in stable 8+ oxidation states, which is among the highest for isolable compounds.
Moving across a period of transition elements, certain patterns in their oxidation states emerge:
- The number of oxidation states of each ion increases up to manganese (group 7), after which they decrease. Later transition metals have a stronger attraction between protons and electrons (because there are more of them present), requiring more energy to remove the electrons.
- When these elements are in lower oxidation states, they can be found as simple ions. In their higher oxidation states, these elements are usually bonded covalently to electronegative elements like oxygen or fluorine, forming polyatomic ions such as chromate, vanadate, or permanganate.
Other properties associated with the stability of oxidation states are as follows:
- Ions in higher oxidation states tend to make good oxidizing agents, whereas elements in low oxidation states become reducing agents.
- Going across a period, the 2+ ions start as strong reducing agents and increase in stability.
- Conversely, the 3+ ions start at higher stability and become more oxidizing across the period.
Colored compounds
The chemistry of transition metals as we have seen is characterized by the partially filled d orbitals allowing multiple oxidation states. Another consequence of the d orbitals for the chemistry of these elements is that they can form stable complexes in coordination compounds. In some of these coordination compounds the transition metal can have a zero or even negative oxidation state. In the complex the transition metal atom or ion forms weak covalent bonds to other small molecules or ions known as ligands.
Transition metal compounds are often highly colored and coordination by ligands plays a large part in determining color of the compound. In the absence of ligands the d orbitals of an atom all have the same energy, but when surrounded by ligands the enegies of the d orbitals change in characteristic ways, and they no longer all have the same energy. This is described by cystal field theory. For many compounds of this type the resulting difference in energy of the d orbitals is in the energy range of visible light. Thus they stongly absorb a particular wavelength of visible light and appear vividly colored. Many different colors can be observed and color even varies between the different ions of a single element. A striking example of this is in the different ions of vanadium (V), VO2+ is yellow in solution, VO2+ is blue, V3+(aq) is green and V2+(aq) is purple.
The color of a complex depends on:
- the nature of the metal ion, specifically the number of electrons in the d orbitals
- the arrangement of the ligands around the metal ion (for example geometric isomers can display different colors)
- the nature of the ligands surrounding the metal ion. The stronger the ligands then the greater the energy difference between the different d orbitals.
Interestingly, though zinc can form complexes they are colorless because the 3d orbitals of zinc are full. The full d orbitals prevent the complex from absorbing visible light when the energies of the d orbitals are altered by the ligands. Zinc is in group 12, so is not considered a transition metal in the newer IUPAC definition
Reference
- ↑ Cotton, F. Albert; Wilkinson, G.; Murillo, C. A. (1999). Advanced Inorganic Chemistry (6th ed.). New York: Wiley.
| Standard table | Vertical table | Table with names | Names and atomic masses (large) | Names and atomic masses (small) | Names and atomic masses (text only) | Inline F-block | Elements to 218 | Electron configurations | Metals and non metals | Table by blocks | List of elements by name |
| Groups: 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 - 9 - 10 - 11 - 12 - 13 - 14 - 15 - 16 - 17 - 18 |
| Periods: 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 |
| Series: Alkalis - Alkaline earths - Lanthanides - Actinides - Transition metals - Poor metals - Metalloids - Nonmetals - Halogens - Noble gases |
| Blocks: s-block - p-block - d-block - f-block - g-block |
| ||||||||
| General subfields within the Natural sciences |
|---|
| Astronomy | Biology | Chemistry | Earth science | Ecology | Physics |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.