Difference between revisions of "Transition metal" - New World Encyclopedia
David Burton (talk | contribs) |
David Burton (talk | contribs) |
||
| Line 113: | Line 113: | ||
The chemistry of transition metals as we have seen is characterized by the partially filled ''d'' orbitals allowing multiple oxidation states. Another consequence of the ''d'' orbitals for the chemistry of these elements is that they can form stable complexes in [[coordination compounds]]. In some of these coordination compounds the transition metal can have a zero or even negative [[oxidation state]]. In the complex the transition metal atom or ion forms weak covalent bonds to other small molecules or ions known as [[ligand]]s. | The chemistry of transition metals as we have seen is characterized by the partially filled ''d'' orbitals allowing multiple oxidation states. Another consequence of the ''d'' orbitals for the chemistry of these elements is that they can form stable complexes in [[coordination compounds]]. In some of these coordination compounds the transition metal can have a zero or even negative [[oxidation state]]. In the complex the transition metal atom or ion forms weak covalent bonds to other small molecules or ions known as [[ligand]]s. | ||
| − | Transition metal compounds are often highly colored and coordination by ligands plays a large part in determining color of the compound. In the absence of ligands the ''d'' orbitals of an atom all have the same energy, but when surrounded by ligands the enegies of the ''d'' orbitals change in characteristic ways, and they no longer all have the same energy. This is described by [[cystal field theory]]. For many compounds of this type the resulting difference in energy of the ''d'' orbitals is in the energy range of visible light. Thus they stongly absorb a particular wavelength of visible light and appear vividly colored. Many different colors can be observed and color even varies between the different ions of a single element | + | Transition metal compounds are often highly colored and coordination by ligands plays a large part in determining color of the compound. In the absence of ligands the ''d'' orbitals of an atom all have the same energy, but when surrounded by ligands the enegies of the ''d'' orbitals change in characteristic ways, and they no longer all have the same energy. This is described by [[cystal field theory]]. For many compounds of this type the resulting difference in energy of the ''d'' orbitals is in the energy range of visible light. Thus they stongly absorb a particular wavelength of visible light and appear vividly colored. Many different colors can be observed and color even varies between the different ions of a single element. A striking example of this is in the different ions of [[vanadium]] (V), <nowiki>VO</nowiki><sub>2</sub><sup>+</sup> is yellow in solution, <nowiki>VO</nowiki><sup>2+</sup> is blue, <nowiki>V</nowiki><sup>3+</sup>(aq) is green and <nowiki>V</nowiki><sup>2+</sup>(aq) is purple. |
The color of a complex depends on: | The color of a complex depends on: | ||
| Line 120: | Line 120: | ||
*the nature of the ligands surrounding the metal ion. The stronger the ligands then the greater the energy difference between the different ''d'' orbitals. | *the nature of the ligands surrounding the metal ion. The stronger the ligands then the greater the energy difference between the different ''d'' orbitals. | ||
| − | Interestingly, though [[zinc]] can form complexes they are colorless because the 3d orbitals of zinc are full. The full ''d'' orbitals prevent the complex from absorbing visible light when the energies of the ''d'' orbitals are altered by the ligands. Zinc is in group 12, so is not considered a transition metal in the newer IUPAC definition | + | Interestingly, though [[zinc]] can form complexes but they are colorless because the 3d orbitals of zinc are full. The full ''d'' orbitals prevent the complex from absorbing visible light when the energies of the ''d'' orbitals are altered by the ligands. Zinc is in group 12, so is not considered a transition metal in the newer IUPAC definition |
== Catalytic activity == | == Catalytic activity == | ||
Revision as of 20:40, 13 June 2006
The elements that divide the main groups of the periodic table in the standard view of the table, that is the elements in groups 3 through 12, are commonly called transition metals or transition elements. The name transition comes from their position in the table. They divide the periods of the table so are a transition between groups 2 and 13. Some of these elements occur naturally in their metallic state and have been known since antiquity. Three of these, gold, silver, and copper are important economically and have been extensively used in coinage and jewelry. Use of copper in tools was one of the first historical technological advances. Transition metals provide some of the important metallic catalysts used in industrial and laboratory settings, and iron in the form of steel is used in many things from cars to bridges.
| The Transition Metals | ||||||||||
| Group | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|
| Period 4 | Sc 21 | Ti 22 | V 23 | Cr 24 | Mn 25 | Fe 26 | Co 27 | Ni 28 | Cu 29 | Zn 30 |
| Period 5 | Y 39 | Zr 40 | Nb 41 | Mo 42 | Tc 43 | Ru 44 | Rh 45 | Pd 46 | Ag 47 | Cd 48 |
| Period 6 | Lu 71 | Hf 72 | Ta 73 | W 74 | Re 75 | Os 76 | Ir 77 | Pt 78 | Au 79 | Hg 80 |
| Period 7 | Lr 103 | Rf 104 | Db 105 | Sg 106 | Bh 107 | Hs 108 | Mt 109 | Ds 110 | Rg 111 | Uub 112 |
Definitions
The general definition of transition metals as groups 3 through 12 (the d-block elements) decribed in the introduction is simple and has been traditionally used. Though this definition is still widely used the characteristic properties of transition metals arise because these elements have partially filled d orbitals. This has lead to a stricter definition of the term. Therefore, more strictly, IUPAC defines the transition metals as any element with an incomplete d subshell or that may form stable ions with an incomplete d subshell (IUPAC definition: "An element whose atom has an incomplete d sub-shell, or which can give rise to cations with an incomplete d sub-shell.").
By this definition, zinc, cadmium, and mercury (group 12) are not considered to be transition metals. This is because their d orbitals are completly filled in both their elemental and stable ionic states. When they form ions they loose only electrons in their outermost s subshell leaving the d subshell intact. Only a few unstable transient species of these elements that leave ions with a partly filled d subshell have been formed.[1] Element 112 may also be excluded since its electronic configuration is likely to be similar to other members of the group 12, and its oxidation properties are unlikely to be observed due to its radioactive nature. Thus this stricter definition of transition metals limits the term to groups 3 to 11.
Properties
There are several common characteristic properties of transition elements:
- They are solids at room temperature (except mercury) with high tensile strength, density, melting and boiling points.
- They are silvery-blue at room temperature (except copper and gold).
- They form monatomic ions with a 2+ charge, but can form ions with more than one charge (i.e. Fe2+ and Fe3+), and often have higher oxidation states in compounds.
- They form complexes in coordination compounds many of which are brightly colored
- They are often good catalysts.
After these common characteristics there are some trends in properties as we go through a period, much like those in the main group elements, but with less dramatic changes. In general atomic radius tends to decrease and first ionization energy increase across the transition metals of a period. Also as we go across the period the metals tend to become softer, and mercury is a liquid at room temperature. Group 11 elements (copper, silver, and gold) are particularly unreactive. These "noble" metals can occur naturally in their elemental metallic state, and are sometimes known as coinage metals as they have been usefull for minting coins.
Electronic configuration
- Main article: electron configuration
The properties of transition metals arise from their defining characteristic of partially filled d orbitals. They are metals because the d orbital electrons are able delocalise within the metal lattice in a metallic bond. Most of the transition metals have two electrons in their outermost s subshell. Then as we go across a period the number of d electons increases by one. So in the fourth period scandium (Sc) has the configuration [Ar]4s23d1, and the next element Titanium (Ti) has the configuration [Ar]4s23d2, etc. There are some exceptions to this, notably copper ([Ar]4s13d10) and chromium ([Ar]4s13d5) in the fourth period. These exceptions occur because half and fully filled subshells impart additional stability. Copper has a completly filled d subshell, and chromium and half filled d subshell. Similar exceptions occur and are more prevalent in the fifth, sixth and seventh period.
When these metals lose electrons to form monatomic ions they generally lose their s electrons first. Thus most transition metals form ions with a 2+ charge. Higher oxidation states involve d electrons as well. Monatomic ions with a charge greater than 3+ are rare, and the higher oxidation states of the transition metals occur in compounds with electronegative elements such as oxygen.
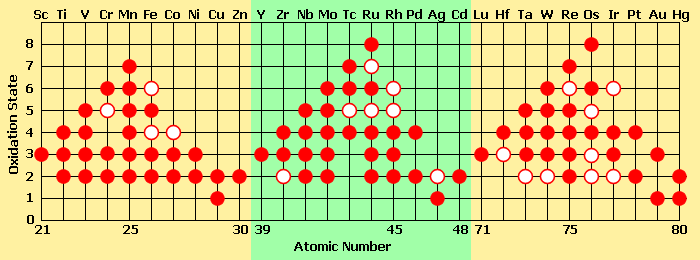
Variable oxidation states
Unlike ions of most main group metals monatomic ions of the transition metals may have more than one stable charge, and in compounds several higher oxidation states. This is because they can lose d electrons without a high energetic penalty. Manganese, for example has two 4s electrons and five 3d electrons, which can be removed. Loss of all of these electrons leads to a 7+ oxidation state. Osmium and ruthenium compounds are commonly isolated in stable 8+ oxidation states, which is among the highest for isolable compounds.
Certain patterns in oxidation state emerge across the period of transition elements:
- The number of oxidation states of each ion increases up to Mn, after which they decrease. Later transition metals have a stronger attraction between protons and electrons (since there are more of each present), which then would require more energy to remove the electrons.
- When the elements are in lower oxidation states, they can be found as simple ions. However transistion metals in higher oxidation states are usually bonded covalently to electronegative elements like oxygen or fluorine, forming polyatomic ions such as chromate, vanadate, or permanganate.
Other properties with respect to the stability of oxidation states:
- Ions in higher oxidation states tend to make good oxidising agents, whereas elements in low oxidation states become reducing agents.
- The 2+ ions across the period start as strong reducing agents and become more stable.
- The 3+ ions start stable and become more oxidizing across the period.
Colored compounds
The chemistry of transition metals as we have seen is characterized by the partially filled d orbitals allowing multiple oxidation states. Another consequence of the d orbitals for the chemistry of these elements is that they can form stable complexes in coordination compounds. In some of these coordination compounds the transition metal can have a zero or even negative oxidation state. In the complex the transition metal atom or ion forms weak covalent bonds to other small molecules or ions known as ligands.
Transition metal compounds are often highly colored and coordination by ligands plays a large part in determining color of the compound. In the absence of ligands the d orbitals of an atom all have the same energy, but when surrounded by ligands the enegies of the d orbitals change in characteristic ways, and they no longer all have the same energy. This is described by cystal field theory. For many compounds of this type the resulting difference in energy of the d orbitals is in the energy range of visible light. Thus they stongly absorb a particular wavelength of visible light and appear vividly colored. Many different colors can be observed and color even varies between the different ions of a single element. A striking example of this is in the different ions of vanadium (V), VO2+ is yellow in solution, VO2+ is blue, V3+(aq) is green and V2+(aq) is purple.
The color of a complex depends on:
- the nature of the metal ion, specifically the number of electrons in the d orbitals
- the arrangement of the ligands around the metal ion (for example geometric isomers can display different colors)
- the nature of the ligands surrounding the metal ion. The stronger the ligands then the greater the energy difference between the different d orbitals.
Interestingly, though zinc can form complexes but they are colorless because the 3d orbitals of zinc are full. The full d orbitals prevent the complex from absorbing visible light when the energies of the d orbitals are altered by the ligands. Zinc is in group 12, so is not considered a transition metal in the newer IUPAC definition
Catalytic activity
Transition metals form good homogeneous or heterogeneous catalysts, for example iron is the catalyst for the Haber process. Nickel, palladium, or platinum can be used in the hydrogenation of alkenes and alkynes. Platinum is the catalyst in the catalytic converters of car exhaust systems.
Reference
- ↑ Cotton, F. Albert; Wilkinson, G.; Murillo, C. A. (1999). Advanced Inorganic Chemistry (6th ed.). New York: Wiley.
| Standard table | Vertical table | Table with names | Names and atomic masses (large) | Names and atomic masses (small) | Names and atomic masses (text only) | Inline F-block | Elements to 218 | Electron configurations | Metals and non metals | Table by blocks | List of elements by name |
| Groups: 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 - 9 - 10 - 11 - 12 - 13 - 14 - 15 - 16 - 17 - 18 |
| Periods: 1 - 2 - 3 - 4 - 5 - 6 - 7 - 8 |
| Series: Alkalis - Alkaline earths - Lanthanides - Actinides - Transition metals - Poor metals - Metalloids - Nonmetals - Halogens - Noble gases |
| Blocks: s-block - p-block - d-block - f-block - g-block |
| ||||||||
| General subfields within the Natural sciences |
|---|
| Astronomy | Biology | Chemistry | Earth science | Ecology | Physics |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.