Norepinephrine
| Norepinephrine[1] | |
|---|---|
| |
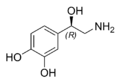
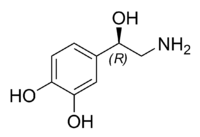
| Chemical name | 4-(2-Amino-1-hydroxyethyl)benzene-1,2-diol |
| Other names | Norepinephrine Noradrenaline |
| C8H11NO3 | |
| Molecular mass | 169.18 g/mol |
| CAS number | D: [149-95-1] L: [51-41-2] D/L: [138-65-8] |
| Density | ? g/cm3 |
| L: 216.5-218 °C (decomp.) D/L: 191 °C (decomp.) | |
| SMILES | OC1=CC=C(C(O)CN)C=C1O |
| Disclaimer and references | |
Norepinephrine or noradrenaline is a hormone and a neurotransmitter, being secreated by the adrenal medulla as a hormone into the blood, and as a neurotransmitter from neurons.
catecholamine and a phenethylamine with chemical formula C8H11NO3. The natural stereoisomer is L-(−)-(R)-norepinephrine. It is released from the medulla of the adrenal glands as a hormone into the blood, but it is also a neurotransmitter in the central nervous system and sympathetic nervous system where it is released from noradrenergic neurons during synaptic transmission. As a stress hormone, it affects parts of the human brain where attention and responding actions are controlled. Along with epinephrine, norepinephrine underlies the fight-or-flight response, directly increasing heart rate, triggering the release of glucose from energy stores, and increasing skeletal muscle readiness.
Norepinephrine is released when a host of physiological changes are activated by a stressful event. This is caused in part by activation of an area of the brain stem called the locus ceruleus. This nucleus is the origin of most norepinephrine pathways in the brain. Neurons that are activated by norepinephrine project bilaterally (send signals to both sides of the brain) from the locus ceruleus along distinct pathways to many locations, including the cerebral cortex, limbic system, and the spinal cord.
At synapses, norepinephrine acts on both alpha and beta adrenoreceptors.
Clinical uses
Attention-deficit/hyperactivity disorder
Norepinephrine, along with dopamine, has come to be recognized as playing a large role in attention and focus. For people with ADD/ADHD, psychostimulant medications such as Ritalin/Concerta (methylphenidate), Dexedrine (dextroamphetamine), and Adderall (a mixture of dextroamphetamine and racemic amphetamine salts) are prescribed to help increase levels of norepinephrine and dopamine. Strattera (atomoxetine) is a selective norepinephrine reuptake inhibitor, and is a unique ADD/ADHD medication, as it affects only norepinephrine, rather than dopamine. As a result, Strattera has a lower abuse potential. However, it may not be as effective as the psychostimulants are with many people who have ADD/ADHD. Consulting with a physician or nurse practitioner is needed to find the appropriate medication and dosage. (Other SNRIs, currently approved as antidepressants, have also been used off-label for treatment of ADD/ADHD.)
Depression
Differences in the norepinephrine system are implicated in depression. Serotonin-norepinephrine reuptake inhibitors (SNRIs) are antidepressants that treat depression by increasing the amount of serotonin and norepinephrine available to postsynaptic cells in the brain. There is some recent evidence showing that the norepinephrine transporter also transports some dopamine as well, implying that SNRIs may also increase dopamine transmission[citation needed]. This is because SNRIs work by inhibiting reuptake, i.e. preventing the serotonin and norepinephrine transporters from taking their respective neurotransmitters back to their storage vesicles for later use. If the norepinephrine transporter normally recycles some dopamine too, then SNRIs will also enhance dopaminergic transmission. Therefore, the antidepressant effects associated with increasing norepinephrine levels may also be partly or largely due to the concurrent increase in dopamine (particularly in the prefrontal cortex).
Tricyclic antidepressants (TCAs) increase norepinephrine as well. Most of them also increase serotonin, but tend to have a lot of side effects due to actions on receptors for histamine and acetylcholine. These include tiredness, increased hunger, dry mouth, and blurred vision. For this reason, they have largely been replaced by newer selective reuptake drugs.
Vasoconstriction
Norepinephrine is also used as a vasopressor medication (for example, brand name Levophed) for patients with critical hypotension. It is given intravenously and acts on both alpha-1 and alpha-2 adrenergic receptors to cause vasoconstriction. Its effect in-vitro is often limited to the increasing of blood pressure through antagonising alpha-1 and alpha-2 receptors and causing a resultant increase in systemic vascular resistance. In high dose and especially when it is combined with other vasopressors, it can lead to limb ischemia and limb death. Norepinephrine is mainly used to treat patients in vasodilatory shock states such as Septic Shock and Neurogenic shock and has shown a survival benefit over dopamine.
Biosynthesis
Norepinephrine is synthesized by a series of enzymatic steps in the adrenal medulla from the amino acid tyrosine:
- The first reaction is the oxidation into dihydroxyphenylalanine (L-DOPA).
- This is followed by decarboxylation into the neurotransmitter dopamine.
- Last is the final β-oxidation into norepinephrine by dopamine beta hydroxylase.
Metabolites
In mammals, norepinephrine is rapidly degraded to various metabolites. The principal metabolites are:
- Normetanephrine (via the enzyme catechol-O-methyl transferase, COMT)
- 3,4-Dihydroxymandelic acid (via monoamine oxidase, MAO)
- 3-Methoxy-4-hydroxymandelic acid (via MAO)
- 3-Methoxy-4-hydroxyphenylglycol (via MAO)
- Epinephrine (via PNMT[2])
ReferencesISBN links support NWE through referral fees
- ↑ Merck Index, 11th Edition, 6612.
- ↑ "Endokrynologia Kliniczna" ISBN 83-200-0815-8, page 502
External links
- http://www.surgeongeneral.gov/library/mentalhealth/chapter4/sec2_1.html
- http://www.med.upenn.edu/astonjoneslab/epapers/A-JNeuropsycho5thGen.pdf
- http://www.biopsychiatry.com/nordop.htm
Template:ChemicalSources
| Phenethylamines edit |
|---|
|
{2C-B} {2C-C} {2C-D} {2C-E} {2C-I} {2C-N} {2C-T-2} {2C-T-21} {2C-T-4} {2C-T-7} {2C-T-8} {3C-E} {4-FMP} {Amphetamine} {Bupropion} {Cathine} {Cathinone} {DESOXY} {Diethylcathinone} {Dimethylcathinone} {DOC} {DOB} {DOI} {DOM} {bk-MBDB} {Dopamine} {Br-DFLY} {Ephedrine} {Epinephrine} {Escaline} {Fenfluramine} {Levalbuterol} {Levmetamfetamine} {MBDB} {MDA} {MDMA} {MDMC/Methylone} {MDEA} {Mescaline} {Methamphetamine} {Methcathinone} {Methylphenidate} {Norepinephrine} {Phentermine} {Salbutamol} {Tyramine} {Venlafaxine} |
| Hormones and endocrine glands - edit |
|---|
|
Hypothalamus: GnRH - TRH - CRH - GHRH - somatostatin - dopamine | Posterior pituitary: vasopressin - oxytocin | Anterior pituitary: GH - ACTH - TSH - LH - FSH - prolactin - MSH - endorphins - lipotropin Thyroid: T3 and T4 - calcitonin | Parathyroid: PTH | Adrenal medulla: epinephrine - norepinephrine | Adrenal cortex: aldosterone - cortisol - DHEA | Pancreas: glucagon- insulin - somatostatin | Ovary: estradiol - progesterone - inhibin - activin | Testis: testosterone - AMH - inhibin | Pineal gland: melatonin | Kidney: renin - EPO - calcitriol - prostaglandin | Heart atrium: ANP Stomach: gastrin | Duodenum: CCK - GIP - secretin - motilin - VIP | Ileum: enteroglucagon | Liver: IGF-1 Placenta: hCG - HPL - estrogen - progesterone Adipose tissue: leptin, adiponectin Target-derived NGF, BDNF, NT-3 |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.