Difference between revisions of "Guinea worm disease" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 55: | Line 55: | ||
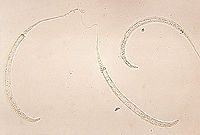
''Dracunculus medinensis'' is a long and very thin [[nematode]] (roundworm) (Andrews and Berger 2006). It is the female guinea worm that causes the symptoms of dracunculiasis (Bimi 20007). The adult female is primarily larger than the adult male. The male guinea worm is typically 12 to 29 mm (0.5 to 1.1 inches) while the female can grow to 0.6 to 0.9 meters (2 to 3 feet) long and be as thick as a [[spaghetti]] noodle (Palmer and Reeder 2005). The longest adult female recorded was {{convert|800|mm|abbr=on}}, while the adult male was only {{convert|40|mm|abbr=on}} (Schmidt and Roberts 2009). The female guinea worm is among the longest nematodes infecting humans (Saleem and Ahmed 2006). | ''Dracunculus medinensis'' is a long and very thin [[nematode]] (roundworm) (Andrews and Berger 2006). It is the female guinea worm that causes the symptoms of dracunculiasis (Bimi 20007). The adult female is primarily larger than the adult male. The male guinea worm is typically 12 to 29 mm (0.5 to 1.1 inches) while the female can grow to 0.6 to 0.9 meters (2 to 3 feet) long and be as thick as a [[spaghetti]] noodle (Palmer and Reeder 2005). The longest adult female recorded was {{convert|800|mm|abbr=on}}, while the adult male was only {{convert|40|mm|abbr=on}} (Schmidt and Roberts 2009). The female guinea worm is among the longest nematodes infecting humans (Saleem and Ahmed 2006). | ||
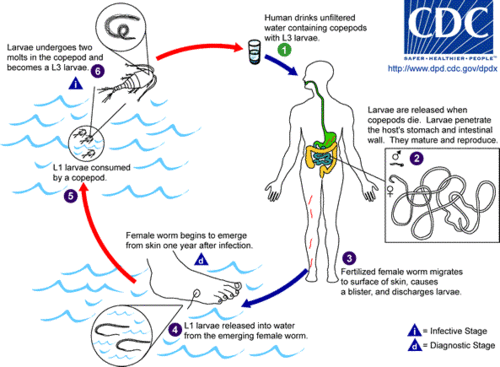
| − | L1 stage larvae are consumed by water fleas ((microscopic [[arthropod]]s known as [[ | + | L1 stage larvae are consumed by water fleas ((microscopic [[arthropod]]s known as [[copepod]]s) in contaminated drinking water. The larvae develop for approximately two weeks inside the copepods, undergoing two molts and becoming L3 stage larvae. At this stage the larvae can cause guinea worm disease if the infected copepods are not filtered from drinking water and are consumed by humans. |
| − | Once inside the body, [[stomach acid]] [[digestion|digests]] and kills the water flea, but not the guinea worm larvae that are sheltered inside. The stage 3 larvae then penetrate the host's [[stomach]] or [[intestinal]] wall, and enter into the [[abdominal cavity]] and [[retroperitoneal space]]. After maturing, the male and female worms mate. This takes place approximately three months after infection. The male worm dies after mating and is absorbed (Palmer and Reeder 2005). | + | Once inside the body, [[stomach acid]] [[digestion|digests]] and kills the water flea, but not the guinea worm larvae that are sheltered inside. The stage 3 larvae then penetrate the host's [[stomach]] or [[intestinal]] wall, and enter into the [[abdominal cavity]] and [[retroperitoneal space]]. After maturing, the male and female worms mate. This takes place approximately three months after infection. The male worm dies after mating and is absorbed (Palmer and Reeder 2005). The female, which contains larvae, burrows into the deeper [[connective tissue]]s or adjacent to [[long bone]]s or joints of the extremities (Palmer and Reeder 2005). |
[[File:Drac life cycle.gif|thumb|left|500px|Life cycle of ''Dracunculus medinensis'']] | [[File:Drac life cycle.gif|thumb|left|500px|Life cycle of ''Dracunculus medinensis'']] | ||
| − | Approximately one year after mating the fertilized females migrate in the [[subcutaneous tissue]]s towards the surface of the skin causing formation of blisters on the skin, generally on the distal lower extremity (foot), | + | Approximately one year after mating, the fertilized females migrate in the [[subcutaneous tissue]]s towards the surface of the skin causing formation of painful, ulcerating blisters on the skin, generally on the distal lower extremity (foot)(CDC 2007). Within 72 hours the blister ruptures, exposing one end of the emergent worm. This blister causes a very painful burning sensation as the worm emerges. The females can also emerge from other parts of the body, such as the head, torso, upper extremities, buttocks, and genitalia (Schmidt and Roberts 2009). The patients then often seek to relieve the burning sensation by placing their foot in water, but when the lesion comes into contact with water, the female worm emerges and releases hundreds of thousands of her stage 1 larvae, contaminating the water supply. |
| + | During the next few days, the female worm is capable of releasing more larvae whenever it comes in contact with water as it extends its posterior end through the hole in the host's skin. These larvae contaminate the water supply and are eaten by [[copepod]]s, then humans, thereby repeating the life-cycle of the disease. Infected copepods can live in the water for only two to three weeks if they are not ingested by a person. | ||
| − | + | Infection does not create immunity, so people can repeatedly experience guinea worm disease throughout their lives (CDC 2007). | |
| − | + | In drier areas just below the Sahara desert, cases of the disease often emerge during the rainy season, which for many agricultural communities is also the planting or harvesting season. Elsewhere, the emerging worms are more prevalent during the dry season, when ponds and lakes are smaller and copepods are thus more concentrated in them. Guinea worm disease outbreaks can cause serious disruption to local food supplies and school attendance (CDC 2007). | |
| − | |||
| − | + | <ref>{{cite journal |author=Langbong Bimi |year=2007 |title=Potential vector species of Guinea worm (''Dracunculus medinensis'') in Northern Ghana |journal=[[Vector-Borne and Zoonotic Diseases]] |volume=7 |issue=3 |pages=324–329 |doi=10.1089/vbz.2006.0622 |pmid=17767406}}</ref> | |
| + | (CDC 2007). <ref name="MMWR">{{cite journal |title=Progress toward global eradication of dracunculiasis, January 2005-May 2007 |journal=MMWR Morb. Mortal. Wkly. Rep. |volume=56 |issue=32 |pages=813–7 |year=2007 |month=August |pmid=17703170 |url=http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5632a1.htm |author1= Centers for Disease Control and Prevention (CDC)}}</ref> | ||
| − | + | <ref name="Schmidt">{{cite book |author=G. D. Schmidt & L S. Roberts |editor=Larry S. Roberts & John Janovy, Jr. |year=2009 |title=Foundations of Parasitology |edition=8th |publisher=[[McGraw-Hill]] |pages=480–484 |isbn=978-0-07-128458-5}}</ref> | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | <ref>{{cite web |publisher=[[Centers for Disease Control and Prevention]] |title=''Dracunculus medinensis'' |year=2009 |url=http://www.dpd.cdc.gov/dpdx/HTML/Dracunculiasis.htm |accessdate=November 25, 2009}}</ref> | |
| − | <ref>{{cite | ||
| − | |||
| − | |||
Revision as of 01:29, 26 July 2013
| Guinea worm disease (Dracunculiasis) | |
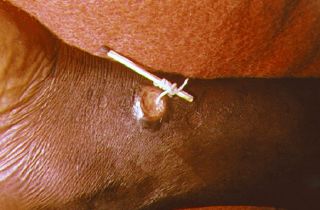 A method used to extract a guinea worm from the leg of a human | |
| ICD-10 | B72 |
|---|---|
| ICD-9 | 125.7 |
| DiseasesDB | 3945 |
| eMedicine | ped/616 |
| MeSH | D004320 |
Guinea worm disease (GWD), also called dracunculiasis, is a parasitic infection caused by the nematode (roundworm) Dracunculus medinensis (guinea worm). The disease is spread by drinking water contaminated by copepods that harbor the guinea worm larvae. The larvae mate in the human body and the females mature and develop into two-to-three-foot-long worms, which often forms a painful lesion on a lower limb and may exit excruciatingly through a leg or foot, among other parts of the human body. GWD is the only human disease known to be contracted exclusively by drinking water.
The guinea worm is one of the best historically documented human parasites, even noted in the ancient Ebers Papyrus. Once widely spread through tropical Africa and Asia, GWD was reported in only four countries in 2012 (Chad, Ethiopia, Mali, and South Sudan); Asia is free of the disease. The incidence of the disease has gone from an estimate of more than 3 million cases a year in 1986 to only 542 reported cases in 2012. It may become the second human disease to be eradicated, after smallpox, and the first parasitic disease eradicated, with the eradication done without vaccines or medical treatment.
Overview
Guinea worm disease is a nodular dermatosis produced by the development of Dracunculus parasite in the subcutaneous tissue of mammals. Dracunculus medinensis has been reported in humans, dogs, cats, horses, cattle, and other animals in Africa and Asia, although James Hughes, professor of medicine and public health at Emory University, states that humans are the only reservoir of the disease (McKeever 2013; Nelson 2012). A similar species of the Dracunculus genus, D. insignis, is a parasite which causes Dracunculiasis in dogs, raccoons, minks, foxes, otters, and skunks of North America (McKeever 2013).
The parasite enters a host by way of host ingestion of stagnant water contaminated with copepods (water fleas) infested with guinea worm larvae. The copepods serve as agents of disease transmission. Approximately a year after ingestion, the disease presents with a painful, burning sensation as the female worm forms a blister, usually on the lower limb. The mature worm, now two to three feet in length, often exits the lower limb or foot and is excruciatingly painful (Nelson 2012)
The guinea worm is one of the best historically documented human parasites, including being mentioned in the Egyptian medical Ebers Papyrus, dating from about 1550 B.C.E. (Palmer and Reeder 2005). Tales of the behavior of the guinea worm can also be found in writings from the 2nd century B.C.E., in accounts penned by Greek chroniclers (Piper 2007). The 2nd century B.C.E., Greek writer Agatharchides, described this affliction as being endemic among certain nomads in what is now Sudan and along the Red Sea (Palmer and Reeder 2005). Guinea worm has been found in calcified Egyptian mummies (Carter Center 2013) and it has been speculated that an Old Testament description of "fiery serpents" may have been referring to guinea worm: "And the Lord sent fiery serpents among the people, and they bit the people; and much people of Israel died" (Numbers 21:4–9) (Palmer and Reeder 2005). The traditional (and still current) method of extracting guinea worm by twisting the worm around a stick may have inspired the rod of Asclepius, a symbol of medicine since Ancient Greek times, which portrays a snake winding around a staff (Blayney 2002).
The name dracunculiasis is derived from the Latin "affliction with little dragons" (Barry 2007), while the common name "guinea worm" appeared after Europeans saw the disease on the Guinea coast of West Africa in the 17th century (Palmer and Reeder 2005). The unusually high incidence of dracunculiasis in the city of Medina led to it being included in part of the disease's scientific name medinensis. Guinea worm is no longer endemic in either location.
Once prevalent in 21 nations in Asia and Africa, with estimates of 3.5 million cases a year in 1986, the disease remains endemic among humans in only four countries in Africa (Carter Center 2013; Nelson 2012). In 2012, only 542 cases were reported (Carter Center 2013).
The Carter Center, which has spearheaded the eradication effort with such partners as the World Health Organization and the Centers for Disease Control, has predicted that guinea worm disease "will be the first parasitic disease to be eradicated and the first disease to be eradicated without the use of vaccines or medical treatment" (Carter Center 2013; Nelson 2012). Former U.S. President Jimmy Carter has been quoted as saying "We are approaching the demise of the last guinea worm who will ever live on earth" (Nelson 2012).
The primary mode of prevention is through behavior change, alongside the provision of clean water sources and the treatment of contaminated drinking water with larvicides. There is no animal or environmental reservoir of D. medinensis and thus the parasite must pass through a host each year to survive (Carter Center 2013).
Guinea worm and life cycle
| Dracunculus medinensis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||||
| Scientific classification | ||||||||||||||||
| ||||||||||||||||
| Dracunculus medinensis (Linnaeus, 1758) | ||||||||||||||||
|
Gordius medinensis Linnaeus, 1758 |
Dracunculus medinensis is a long and very thin nematode (roundworm) (Andrews and Berger 2006). It is the female guinea worm that causes the symptoms of dracunculiasis (Bimi 20007). The adult female is primarily larger than the adult male. The male guinea worm is typically 12 to 29 mm (0.5 to 1.1 inches) while the female can grow to 0.6 to 0.9 meters (2 to 3 feet) long and be as thick as a spaghetti noodle (Palmer and Reeder 2005). The longest adult female recorded was 800 mm (31 in), while the adult male was only 40 mm (1.6 in) (Schmidt and Roberts 2009). The female guinea worm is among the longest nematodes infecting humans (Saleem and Ahmed 2006).
L1 stage larvae are consumed by water fleas ((microscopic arthropods known as copepods) in contaminated drinking water. The larvae develop for approximately two weeks inside the copepods, undergoing two molts and becoming L3 stage larvae. At this stage the larvae can cause guinea worm disease if the infected copepods are not filtered from drinking water and are consumed by humans.
Once inside the body, stomach acid digests and kills the water flea, but not the guinea worm larvae that are sheltered inside. The stage 3 larvae then penetrate the host's stomach or intestinal wall, and enter into the abdominal cavity and retroperitoneal space. After maturing, the male and female worms mate. This takes place approximately three months after infection. The male worm dies after mating and is absorbed (Palmer and Reeder 2005). The female, which contains larvae, burrows into the deeper connective tissues or adjacent to long bones or joints of the extremities (Palmer and Reeder 2005).
Approximately one year after mating, the fertilized females migrate in the subcutaneous tissues towards the surface of the skin causing formation of painful, ulcerating blisters on the skin, generally on the distal lower extremity (foot)(CDC 2007). Within 72 hours the blister ruptures, exposing one end of the emergent worm. This blister causes a very painful burning sensation as the worm emerges. The females can also emerge from other parts of the body, such as the head, torso, upper extremities, buttocks, and genitalia (Schmidt and Roberts 2009). The patients then often seek to relieve the burning sensation by placing their foot in water, but when the lesion comes into contact with water, the female worm emerges and releases hundreds of thousands of her stage 1 larvae, contaminating the water supply.
During the next few days, the female worm is capable of releasing more larvae whenever it comes in contact with water as it extends its posterior end through the hole in the host's skin. These larvae contaminate the water supply and are eaten by copepods, then humans, thereby repeating the life-cycle of the disease. Infected copepods can live in the water for only two to three weeks if they are not ingested by a person.
Infection does not create immunity, so people can repeatedly experience guinea worm disease throughout their lives (CDC 2007).
In drier areas just below the Sahara desert, cases of the disease often emerge during the rainy season, which for many agricultural communities is also the planting or harvesting season. Elsewhere, the emerging worms are more prevalent during the dry season, when ponds and lakes are smaller and copepods are thus more concentrated in them. Guinea worm disease outbreaks can cause serious disruption to local food supplies and school attendance (CDC 2007).
(CDC 2007). [2]
Signs and symptoms
As the worm moves downwards, usually to the lower leg, through the subcutaneous tissues it leads to intense pain localized to its path of travel. The painful, burning sensation experienced by infected people has led to the disease being called "the fiery serpent." Other symptoms include fever, nausea, and vomiting.[6]
Prevention
Guinea worm disease can be transmitted only by drinking contaminated water, and can be completely prevented through two relatively simple measures:[6]
1. Preventing people from drinking Cyclops-contaminated water. The Cyclops (copepod) (water fleas) can be seen in clear water as swimming white specks.
- Drinking water drawn only from sources that are free from contamination, such as a borehole or wells.
- Filtering drinking water, using a fine-mesh cloth filter like nylon, to remove the guinea worm-containing crustaceans. Even folding regular cotton cloth over a few times is an effective filter.
- Filtration through ceramic or sand filters
- Boiling
- Developing new sources of drinking water that lack the parasites, or repairing dysfunctional ones.
- Treating water sources with larvicides to kill the Cyclops[7]
2. Preventing people with emerging Guinea worms from entering water sources used for drinking.
- Community-level case detection and containment is key. This requires staff going door to door looking for cases and a population willing to help and not hide their cases.
- Controlled immersion of emerging worms in buckets of water to reduce the number of larva in the individual worms, followed by discarding the water on dry ground.
- Discouraging all members of the community from setting foot in the drinking water source
- Employing guards at local water sources to prevent people with emerging worms from entering
Treatment
There is no vaccine or medicine to treat or prevent Guinea worm disease.[6] Once a Guinea worm begins emerging, the first step is to do a controlled submersion of the affected area in a bucket of water. This causes the worm to discharge many of its larva, making it less infectious. The water is then discarded on the ground far away from any water source. Submersion results in subjective relief of the burning sensation and makes subsequent extraction of the worm easier. To extract the worm, a person must wrap the live worm around a piece of gauze or a stick. The process can be long, taking anywhere from hours to months. Gently massaging the area around the blister can help loosen the worm up a bit.[8] This is nearly the same treatment that is noted in the famous ancient Egyptian medical text, the Ebers papyrus from 1550 B.C.E.[9] Some people have said that extracting a Guinea worm feels like the afflicted area is on fire.[10][11] However, if the infection is identified before an ulcer forms, the worm can also be surgically removed by a trained doctor in a medical facility.[12]
Although Guinea worm disease is usually not fatal, the wound where the worm emerges could develop a secondary bacterial infection such as tetanus, which may be life-threatening—a concern in endemic areas where there is typically limited or no access to health care.[13] Analgesics can be used to help reduce swelling and pain and antibiotic ointments can help prevent secondary infections at the wound site.[12] At least in the Northern region of Ghana, the Guinea worm team found that antibiotic ointment on the wound site caused the wound to heal too well and too quickly making it more difficult to extract the worm and more likely that pulling would break the worm. The local team preferred to use something called "Tamale oil" (after the regional capital) which lubricated the worm and aided its extraction. As a practical matter, many patients were also given prophylactic oral antibiotics.[citation needed]
It is of great importance not to break the worm when pulling it out. Broken worms have a tendency to putrefy or petrify. Putrefaction leads to the skin sloughing off around the worm. Petrification is a problem if the worm is in a joint or wrapped around a vein or other important area.
Use of metronidazole or thiabendazole may make extraction easier, but also may lead to migration to other parts of the body.[14]
Epidemiology
In 1986, there were an estimated 3.5 million cases of Guinea worm in 20 endemic nations in Asia and Africa.[8] Ghana alone reported 180,000 cases in 1989. The number of cases has since been reduced by more than 99.98% to 542 in 2012[15] - in the four remaining endemic nations of Africa: South Sudan, Chad, Mali and Ethiopia. This is the lowest number of cases since the eradication campaign began. As of 2010, however, the WHO predicted it will be "a few years yet" before eradication is achieved, on the basis that it took 6–12 years for the countries that have so far eliminated Guinea worm transmission to do so after reporting a similar number of cases to that reported in southern Sudan (now South Sudan) in 2009.[16]
The World Health Organization is the international body that certifies whether a disease has been eliminated from a country or eradicated from the world.[17] The Carter Center also reports the status of the Guinea worm eradication program by country.[18]
Certified free
Endemic countries must report to the International Commission for the Certification of Dracunculiasis Eradication and document the absence of indigenous cases of Guinea worm disease for at least three consecutive years to be certified as Guinea worm-free by the World Health Organization.[19]
The results of this certification scheme have been remarkable: by 2007, Benin, Burkina Faso, Chad, Côte d'Ivoire, Kenya, Mauritania, Togo, and Uganda had stopped transmission, and Cameroon, CAR, India, Pakistan, Senegal, Yemen were WHO certified.[18]
Endemic
At the end of 2012, South Sudan, Mali, Ethiopia and Chad still had endemic transmission. The major focus is South Sudan (independent after 2011, formerly the southern region of Sudan), which reported 96% of all cases in 2012.[20]
| Country | Location | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | Description |
|---|---|---|---|---|---|---|---|---|
| South Sudan | 100px | 5,815[21] | 3,618[21] | 2,733[16] | 1,698[22] | 1,028[23] | 521[23] | Sudan increased its efforts at eradication from 2005 when the Comprehensive Peace Agreement was signed, ending a more than two-decade long civil war. 2006 saw an increase to 15,539 cases of Guinea Worm disease, from 5,569 cases in 2005, as a result of reporting from endemic areas that were no longer war-torn. The Southern Sudan Guinea Worm Eradication Program (SSGWEP) has deployed over 28,000 village volunteers, supervisors and other health staff to work on the program full-time. The SSGWEP was able to slash the number of cases reported in 2006 by 63% to 5,815 cases in 2007. Since 2011, at the time that South Sudan became independent, its northern neighbor Sudan had reported no endemic cases of dracunculiasis .[24] |
| Mali | 100px | 313[21] | 417[21] | 186[16] | 57[22] | 12[23] | 7[23] | Four of Mali's regions—(Kayes, Koulikoro, Ségou, and Sikasso)—have eliminated dracunculiasis, while the disease is still endemic in the country's other four regions (Gao, Kidal, Mopti, and Timbuktu). Late detection of two outbreaks, due to inadequate surveillance, in 2007 resulted in a meager 36% containment rate in Mali in 2007.[24] 2008 and 2009 were more successful, however, with containment rates of 85% and 73% respectively.[25] The civil war prevented accurate information from being gathered in northern Mali in 2012. |
| Ethiopia | 100px | 0[21] | 41[21] | 24[16] | 21[22] | 8[23] | 4[23] | Prior to March 2008, there had been no cases reported in Ethiopia since June 2006.[26] |
| Chad | 100px | 0[27] | 0[27] | 0[27] | 10[22] | 10[23] | 10[23] | Prior to 2010, Chad had not reported any indigenous cases of guinea worm in over 10 years.[27] |
| Ghana | 
|
3,358[21] | 501[21] | 242[16] | 8[22] | 0[23] | 0 | In Ghana, after a decade of frustration and stagnation, in 2006 a decisive turnaround was achieved. Multiple changes can be attributed to the improved containment and lower incidence of dracunculiasis: better supervision and accountability, active oversight of patients daily by paid staff, and an intensified public awareness campaign. After Jimmy Carter's visit to Ghana in August 2006, the government of Ghana declared Guinea worm disease to be a public health emergency. The overall rate of contained cases has increased in Ghana from 60% in 2005, to 75% in 2006, 84% in 2007, 85% in 2008, 93% in 2009, and 100% in 2010.[24][25][27] |
2011
Ghana appears to have eradicated guinea worm. In August 2011, their public health officials reported that the country was free of reported cases for over 14 months.[28] While promising, given the incubation period, it will be some time before the WHO certifies Ghana as free of this disease.
To the end of June 2011 the number of cases were: Chad 2 cases; Ethiopia 6 cases; Mali 3 cases: and South Sudan 806 cases. After the separation of South Sudan from Northern Sudan, the territory of Northern Sudan has been free of guinea worm disease for several years and it has been certified free of this disease by the WHO.
To the end of August 2011 the number of cases reported were: Chad 7 cases; Ethiopia 8 cases; Mali 9 cases: and South Sudan 944 cases. These occurred in a total of 440 villages. One possible case has been reported from India: investigation into this is ongoing. India has been certified free since 2000 and it is suspected that this possible case may be an imported one. The majority of cases (78%) in South Sudan were located in adjacent two counties of the Eastern Equatoria state: Kapoeta East County (561 cases) and Kapoeta North County (146 cases). Aside from 2 cases imported from South Sudan, no cases were reported in Ethiopia in July and August from the single focus (Gog District) from which all cases in the last few years have been found. Although it is now possible that transmission in Ethiopia has been interrupted official confirmation will not be forthcoming for several months.[29]
Up to September 2011 the number of uncontained cases were: South Sudan - 241; Chad - 6; Mali - 6; Ethiopia - 1. Of the 70 counties in South Sudan, 56 (80%) are considered free of dracunculiasis.
The total number of cases in 2011 was 1060. Of these 1030 were reported from South Sudan. Mali reported 12; Chad reported 10; and Ethiopia 8. The cases in Chad were part of an outbreak that was originally identified in 2010 as part of a pre certification process. Chad had not reported any cases between 2001 and 2009. Two of the cases in Ethiopia were imported from South Sudan.
2012
According to the WHO the number of reported cases of dracunculiasis has continued to drop to 143 cases between 1 January and 30 April 2012 compared with 382 cases during the same period in 2011. South Sudan alone reported 142 cases, or 99% of the global total, and Ethiopia reported 1 case.[30]
The July 16 report from the WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis states that the number of reported cases between 1 January and 30 June 2012 has dropped to 391 from 807 cases in the same period in 2011 - a 52% improvement. By country the number of reported cases are South Sudan 387, Ethiopia 2, Mali 1 and Chad 1. In the same period of 2011 the number of reported cases were South Sudan 794, Ethiopia 8, Mali 3 and Chad 2.[31]
The September 12 report from the WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis states that the number of reported cases between 1 January and 31 August 2012 has dropped to 484 from 944 cases in the same period in 2011 - a 49% improvement. By country the number of reported cases are South Sudan 494, Ethiopia 3, Mali 4 and Chad 7. In the same period of 2011 the number of reported cases were South Sudan 944, Ethiopia 6, Mali 9 and Chad 8. However, since March 2012, Mali’s Guinea Worm Eradication Program workers have had limited access to the rebel–held regions in northern Mali, and are not able to investigate cases there. Médecins du Monde have reported rumours of a further 5 cases in northern Mali since March, but these have not been confirmed, nor included in the figures.[32]
The October 18 report from the WHO Collaborating Center for Research, Training and Eradication of Dracunculiasis states that the number of reported cases between 1 January and 30 September 2012 has dropped to 521 from 1004 cases in the same period in 2011 - a 48% improvement. By country the number of reported cases are South Sudan 502, Ethiopia 3, Mali 7 and Chad 9. In the same period of 2011 the number of reported cases were South Sudan 980, Ethiopia 6, Mali 10 and Chad 8. Again, Mali’s Guinea Worm Eradication Program workers have limited access to northern Mali, and are not able to investigate or confirm all cases there.[33]
The provision figures for all of 2012 are: total 542; South Sudan 521; Chad 10; Mali 7; and Ethiopia 4. This is a 49% improvement of the total compared to 2011.[15]
2013
Up to the end of March, six cases have been reported, all in South Sudan, compared to 61 cases in 2012 in the same period - a 90% reduction in cases.[34] The February WHO report goes on to say after recording no new cases in January "This is not the end of Guinea worm disease - there will be cases later in 2013 - but we can see the end from here."[20] The report also compares the 521 cases in South Sudan in 2012 ( out of 542 globally ) with Ghana's 501 cases in 2008, and says it took 18 more months to subsequently eliminate Dracunculiasis from Ghana, with the last case in May 2010.
By 22 April, three cases have been confirmed in Chad. After 10 years without any reported cases the reporting of 10 cases in each of 2010, 2011 and 2012 is a very unusual pattern. There are concerns that surveillance vigilance has decreased, and renewed efforts are being made to increase monitoring. This includes coordinating monitoring with its neighbors Nigeria and Cameroon.[34]
By the end of May 67 cases have been reported - 55 in South Sudan, 4 in Chad, 3 in Mali and 5 in Ethiopia. No further cases have been reported to 10 June. [35]
History of the eradication program
The global campaign to eradicate Guinea worm disease began at the U.S. Centers for Disease Control and Prevention (CDC) in 1980. In 1986, former U.S. President Jimmy Carter and his not-for-profit organization, The Carter Center, began leading the global campaign, in conjunction with CDC, UNICEF, and WHO.[36] At this time India, Pakistan, Yemen and 17 countries in Africa were endemic for this disease and reported a total of 3.5 million cases per year.
Carter made a personal visit to a Guinea worm endemic village in 1988. He said: "Encountering those victims first-hand, particularly the teenagers and small children, propelled me and Rosalynn to step up the Carter Center's efforts to eradicate Guinea worm disease."[37]
President Carter also recruited two African former heads of state to the battle against Guinea worm disease. Then-former head of state of Mali, General Amadou Toumani Toure (since elected President of Mali) has been a strong advocate of Guinea worm eradication in Mali and all other French-speaking African endemic countries since 1992.[38][39] Since 1999, former Nigerian head of state General (Dr.) Yakubu Gowan has played a similar role in Nigeria, which at the eradication campaign's start had more cases than any other country.[40]
Since humans are the principal host for Guinea worm, and there is no evidence that D. medinensis has ever been reintroduced to humans in any formerly endemic country as the result of non-human infections, the disease can be controlled by identifying all cases and modifying human behavior to prevent it from recurring.[8][41] Once all human cases are eliminated, the disease cycle will be broken, resulting in its eradication.[8]
In 1991, the World Health Assembly (WHA) agreed that Guinea worm disease should be eradicated.[13] At this time there were 400,000 cases reported each year. The Carter Center has continued to lead the eradication efforts, primarily through its Guinea Worm Eradication Program.[42] Other major actors in the eradication of Guinea worm disease include: World Health Organization, U.S. Centers for Disease Control and Prevention, Bill & Melinda Gates Foundation, and UNICEF,[42][43] but the global coalition now includes dozens of other donors, nongovernmental organizations, and institutions, most especially the ministries of health of the affected countries themselves.
In December 2008, The Carter Center announced new financial support totaling US$55 million from the Bill & Melinda Gates Foundation and the United Kingdom Department for International Development.[44] The funds will help address the higher cost of identifying and reporting the last cases of Guinea worm disease. According to The Carter Center, surveillance of countries, including the smallest communities in the most remote areas, needs to be intensified to prevent outbreaks and setbacks. In the case of Guinea worm disease, which has a one-year incubation period, there is a very high cost of maintaining a broad and sensitive monitoring system and providing a rapid response when necessary.[44]
On 30 January 2012 the WHO meeting at the Royal College of Physicians in London launched the most ambitious and largest coalition health project ever, known as London Declaration on Neglected Tropical Diseases which aims to end/control dracunculiasis by 2020, among other diseases.[45] This project was declared under the official support of all major pharmaceutical companies, the Bill & Melinda Gates Foundation, the governments of the United States, United Kingdom DFID and United Arab Emirates and the World Bank.[46]
Barriers
The eradication of Guinea worm disease has faced several challenges:
- Inadequate security in some endemic countries
- Lack of political will from the leaders of some of the countries in which the disease is endemic
- The need for change in behavior in the absence of a magic bullet treatment like a vaccine or medication
- Inadequate funding at certain times[47]
One of the most significant challenges facing Guinea worm eradication has been the civil war in southern Sudan, which was largely inaccessible to health workers due to violence.[47][48] To address some of the humanitarian needs in southern Sudan, in 1995, the longest ceasefire in the history of the war was achieved through negotiations by Jimmy Carter.[47][48] Commonly called the "Guinea worm cease-fire," both warring parties agreed to halt hostilities for nearly six months to allow public health officials to begin Guinea worm eradication programming, among other interventions.[48][49]
Public health officials cite the formal end of the war in 2005 as a turning point in Guinea worm eradication because it has allowed health care workers greater access to southern Sudan's endemic areas.[43] One remaining area in West Africa outside of Ghana remains challenging to ending Guinea worm: northern Mali, where Tuareg rebels have made some affected areas unsafe for health workers.
One of the greater challenges in eradicating dracunculiasis has been and will continue to be in South Sudan (formerly southern Sudan), particularly political uncertainty in the country with national elections in 2009 and the referendum on the status of southern Sudan in 2011 resulting in South Sudan's independence. Sporadic insecurity or widespread civil conflict could at any time ignite, thwarting eradication efforts.[24] The remaining endemic communities in South Sudan are remote, poor and devoid of infrastructure, presenting significant hurdles for effective delivery of interventions against disease. Moreover, residents in these communities are nomadic, moving seasonally with cattle in pursuit of water and pasture, making it very difficult to know where and when transmission occurred. The peak transmission season coincides with the rainy season, hampering travel by public health workers.[50]
Society and culture
The pain caused by the worm's emergence—which typically occurs during planting and harvesting seasons—prevents many people from working or attending school for as long as three months. In heavily burdened agricultural villages fewer people are able to tend their fields or livestock, resulting in food shortages and lower earnings.[8][43] A study in southeastern Nigeria, for example, found that rice farmers in a small area lost US$20 million in just one year due to outbreaks of Guinea worm disease.[8]
Some notes to possibly incorporate
The Russian scientist Aleksej Fedchenko (1844 - 1873) during the 1860s while living in Samarkand was provided with a number of specimens of the worm by a local doctor which he kept in water. While examining the worms Fedchenko noted the presence of water fleas with embryos of the guinea worm within them.
In modern times, the first to describe dracunculiasis and its pathogenesis was the Bulgarian physician Hristo Stambolski, during his exile in Yemen (1877–1878).[51] His theory was that the cause was infected water which people were drinking.
ReferencesISBN links support NWE through referral fees
- ↑ Langbong Bimi (2007). Potential vector species of Guinea worm (Dracunculus medinensis) in Northern Ghana. Vector-Borne and Zoonotic Diseases 7 (3): 324–329.
- ↑ (August 2007). Progress toward global eradication of dracunculiasis, January 2005-May 2007. MMWR Morb. Mortal. Wkly. Rep. 56 (32): 813–7.
- ↑ G. D. Schmidt & L S. Roberts (2009). in Larry S. Roberts & John Janovy, Jr.: Foundations of Parasitology, 8th, McGraw-Hill, 480–484. ISBN 978-0-07-128458-5.
- ↑ Dracunculus medinensis. Centers for Disease Control and Prevention (2009). Retrieved November 25, 2009.
- ↑ Talha Bin Saleem & Irfan Ahmed (2006). "Serpent" in the breast. Journal of Ayub Medical College Abbottabad 18 (4): 67–68.
- ↑ 6.0 6.1 6.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedWHO2010 - ↑ Template:Cite pmid
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 Cite error: Invalid
<ref>tag; no text was provided for refs namedGWEP - ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedTropMed - ↑ World moves closer to eradicating ancient worm disease. World Health Organization (2007-03-27). Retrieved 2008-07-15.
- ↑ McNeil, DG, "Dose of Tenacity Wears Down a Horrific Disease", New York Times, 2006-03-26. Retrieved 2008-07-15.
- ↑ 12.0 12.1 Fact Sheet:Dracunculiasis—Guinea Worm Disease. CDC (2008-07-15). Retrieved 2010-07-12.
- ↑ 13.0 13.1 (December 1993). Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep 42 (RR–16): 1–38.
- ↑ eMedicine article/997617
- ↑ 15.0 15.1 http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/216.pdf
- ↑ 16.0 16.1 16.2 16.3 16.4 WHO (7 May 2010). Dracunculiasis eradication – global surveillance summary, 2009. Wkly Epidemiol Rec 85 (19): 166–175.
- ↑ WHO certifies seven more countries as free of guinea-worm disease. World Health Organization. Retrieved 2010-05-14.
- ↑ 18.0 18.1 Activities by Country - Guinea Worm Eradication Program. Carter Center. Retrieved 2010-03-16. Cite error: Invalid
<ref>tag; name "GWEP Activities" defined multiple times with different content - ↑ CDC (2000-10-11). Progress Toward Global Dracunculiasis Eradication, June 2000. MMWR Morb. Mortal. Wkly. Rep. 49 (14): 731–5.
- ↑ 20.0 20.1 http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/217.pdf
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 21.6 21.7 Progress toward Global Eradication of Dracunculiasis, January 2008–June 2009. Medscape. Retrieved 2010-05-02.
- ↑ 22.0 22.1 22.2 22.3 22.4 WHO (4 March 2011). Monthly report on dracunculiasis cases, January–December 2010. Wkly Epidemiol Rec 86 (10): 91–2.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 23.8 http://www.cartercenter.org/health/guinea_worm/mini_site/current.html
- ↑ 24.0 24.1 24.2 24.3 Hopkins DR, Ruiz-Tiben E, Downs P, Withers PC, Roy S (October 2008). Dracunculiasis eradication: neglected no longer. Am. J. Trop. Med. Hyg. 79 (4): 474–9.
- ↑ 25.0 25.1 Guinea worm wrap-up 195, 12 March 2010 (PDF). Carter Center. Retrieved 2010-03-16.
- ↑ (October 2008). Update: progress toward global eradication of dracunculiasis, January 2007–June 2008. MMWR Morb. Mortal. Wkly. Rep. 57 (43): 1173–6.
- ↑ 27.0 27.1 27.2 27.3 27.4 Guinea Worm Wrap-Up 202 (PDF). Carter Center. Retrieved 2011-01-20.
- ↑ http://www.voanews.com/english/news/africa/west/Ghana-joins-14-other-African-Nations-in-Eradicating-Guinea-Worm-126700018.html
- ↑ http://cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/207.pdf
- ↑ http://www.who.int/dracunculiasis/en/
- ↑ http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/213.pdf
- ↑ http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/214.pdf
- ↑ http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/215.pdf
- ↑ 34.0 34.1 http://www.cartercenter.org/resources/pdfs/news/health_publications/guinea_worm/wrap-up/218.pdf
- ↑ http://www.cartercenter.org/health/guinea_worm/mini_site/current.html
- ↑ International Task Force for Disease Eradication—Original Members (1989–1992).. Carter Center. Retrieved 2008-07-17.
- ↑ (2008-03-31)A Village Woman's Legacy.. TIME.
- ↑ Jimmy Carter (2004-05-19). "Remarks of Mr Jimmy Carter, former President of the United States of America, at the World Health Assembly.".
- ↑ Mali's President Touré, Southern Sudan Program Director Logora Honored With The Jimmy and Rosalynn Carter Award.. Carter Center (2008-04-02). Retrieved 2008-07-17.
- ↑ Jimmy Carter and General Dr. Yakubu Gowon Encourage Nigerian Officials to Control Schistosomiasis, Other Diseases.. Carter Center (2008-04-02). Retrieved 2008-07-17.
- ↑ BIMI, L. and A. R. FREEMAN, M. L. EBERHARD, E. RUIZ-TIBEN, and N. J. PIENIAZEK (10 May 2005). Differentiating Dracunculus medinensis from D. insignis, by the sequence analysis of the 18S rRNA gene. Annals of Tropical Medicine & Parasitology 99 (5): 511–517.
- ↑ 42.0 42.1 2006 Gates Award for Global Health: The Carter Center. Carter Center (2006). Retrieved 2010-03-08.
- ↑ 43.0 43.1 43.2 Donald R. Hopkins, Ernesto Ruiz-Tiben, Philip Downs, P. Craig Withers, Jr., James H. Maguire (2005-10-01). Dracunculiasis Eradication: The Final Inch. American Journal of Tropical Medicine and Hygiene 73 (4): 669–675.
- ↑ 44.0 44.1 Guinea Worm Cases Hit All-Time Low: Carter Center, WHO, Gates Foundation, and U.K. Government Commit $55 Million Toward Ultimate Eradication Goal. Carter Center. Retrieved 2008-12-08.
- ↑ Uniting to Combat Neglected Tropical Diseases (30 January 2012). London Declaration on Neglected Tropical Diseases. Uniting to Combat NTDs. Retrieved 2013-05-06.
- ↑ WHO (3 February 2012). WHO roadmap inspires unprecedented support to defeat neglected tropical diseases. World Health Organization. Retrieved 2013-05-06.
- ↑ 47.0 47.1 47.2 Cite error: Invalid
<ref>tag; no text was provided for refs namedBarry - ↑ 48.0 48.1 48.2 Sudan. Carter Center. Retrieved 2008-07-15.
- ↑ Hopkins, Donald R. (2002). Sudan's war and eradication of dracunculiasis. Lancet 360: s21–2.
- ↑ WHO (2 May 2008). Dracunculiasis eradication. Wkly Epidemiol Rec 83 (18): 159–167.
- ↑ Христо Стамболски: Автобиография, дневници и mспомени. (Autobiography of Hristo Stambolski. Sofia : Dŭržavna pečatnica, 1927–1931)
.[4]
- McKeever Dermatology Clinics. 2013. Dracunculiasis. Mckeever Dermatology Clinics. Retrieved July 24, 2013.
- Nelson, R. 2012. The last worm: A dreaded tropical disease is on the verge of eradication. Scientific American 302(1):24.
- Palmer, P. E. S., and M. M. Reeder. 2005. Guinea Worm Infection (Dracunculiasis). In Palmer and Reeder's, The Imaging of Tropical Disease (DVD, Internet version)]. Reproduced from the Original Text by The Uniformed Services University of the Health Sciences, and Distributed by The American College of Radiology, the RSNA, the Radiology Outreach Foundation, and The International Society of Radiology With the Kind Permission of Springer Publishing, the Authors, and the Above Institutions. Retrieved July 24, 2013.
- Piper, R. 2007. Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Press. ISBN 9780313339226.
External links
- Guinea Worm Disease Eradication Program. Carter Center.
- Nicholas D. Kristof from the New York Times follows a young Sudanese boy with a Guinea Worm parasite infection who is quarantined for treatment as part of the Carter programme
- Tropical Medicine Central Resource: "Guinea Worm Infection (Dracunculiasis)"
- World Health Organization on Dracunculiasis
Template:Helminthiases
Template:Diseases of Poverty
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ James, WD (2006). Andrews' Diseases of the Skin: Clinical Dermatology. Saunders Elsevier. ISBN 0-7216-2921-0.
- ↑ Barry M (June 2007). The tail end of guinea worm—global eradication without a drug or a vaccine. N. Engl. J. Med. 356 (25): 2561–4.
- ↑ Keith Blayney (2002). Caduceus vs Staff of Asclepius. Retrieved 2009-06-24.
- ↑ Guinea Worm Eradication Program. The Carter Center. Carter Center. Retrieved 2011-03-01.
- ↑ Tropical Medicine Central Resource. Dracunculiasis. Uniformed Services University of the Health Sciences. Retrieved 2008-07-15.