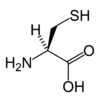
Cysteine
| |
Cysteine | |
| Systematic (IUPAC) name | |
| (2R)-2-amino-3-sulfanyl-propanoic acid | |
| Identifiers | |
| CAS number | 52-90-4 |
| PubChem | 5862 |
| Chemical data | |
| Formula | C3H7NO2S |
| Mol. weight | 121.16 |
| Complete data | |
Cysteine is a naturally occurring, sulfur-containing amino acid that is found in most proteins, although only in small quantities. Cysteine is unique amongst the twenty natural amino acids as it contains a thiol group. Thiol groups can undergo oxidation/reduction (redox) reactions; when cysteine is oxidised it can form cystine, which is two cysteine residues joined by a disulfide bond. This reaction is reversible: as reduction of this disulphide bond regenerates two cysteine molecules. The disulphide bonds of cystine are crucial to defining the structures of many proteins.
Cysteine is often involved in electron-transfer reactions, and help the enzyme catalyse its reaction. Cysteine is also part of the antioxidant glutathione.
N-acetyl-L-cysteine (NAC) is a form of cysteine where an acetyl group is attached to cysteine's nitrogen atom and is sold as a dietary supplement. Cysteine is named after cystine, which comes from the Greek word kustis meaning bladder − cystine was first isolated from kidney stones.
Reactions
As cysteine contains a sulphydryl group, it can undergo redox reactions. Oxidation of cysteine can produce a disulfide bond with another thiol, or further oxidation can produce sulphfinic or sulfonic acids.
The cysteine thiol group is also a nucleophile and can undergo addition and substitution reactions. Thiol groups become much more reactive when they are ionised, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell.[1]
The thiol group also has a high affinity for heavy metals and proteins containing cysteine will bind metals such as mercury, lead and cadmium tightly.[2]
Biological functions
Due to this ability to undergo redox reactions, cysteine has antioxidant properties. Cysteine is an important source of sulfur in human metabolism, and although it is classified as a non-essential amino acid, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine may at some point be recognized as an essential or conditionally essential amino acid.[citation needed]
Cysteine and Glutathione: Cysteine is an important precursor in the production of glutathione in the body and other organisms. The systemic availability of oral glutathione (GSH) is negligible; the vast majority of it must be manufactured intracellularly. Glutathione is a tripeptide antioxidant made up of the three amino acids cysteine, glycine and glutamate. Glutamate and glycine are readily available in most North American diets, but the availability of cysteine makes it be the rate-limiting substrate for the synthesis of glutathione within the cell. It is the sulfhydryl (thiol) group (SH) of cysteine that serves as proton-donor and is responsible for the biological activity of glutathione.[3]
Absorption: The free amino acid cysteine (supplied supplementally by NAC) does not represent an ideal delivery system to the cell. Cysteine is potentially toxic and is spontaneously catabolized in the gastrointestinal tract and blood plasma. Conversely, cysteine absorbed during digestion as cystine (two cysteine molecules linked by a disulfide bond) in the gastrointestinal tract is more stable than the free amino acid cysteine. Cystine travels safely through the GI tract and blood plasma and is promptly reduced to the two cysteine molecules upon cell entry.[3]
Biochemistry
Cysteine contains a thiol group, which can display nucleophilicity. Some important cysteine-derived nucleophiles include ubiquitin ligases, which transfer ubiquitin to its pendant proteins, and caspases, which engage in proteolysis in the apoptotic cycle. Inteins often function with the help of a catalytic cysteine. These roles are typically limited to the intracellular milieu, where the environment is reducing, and cysteine is not oxidized to cystine.
Cysteines play a valuable role by crosslinking proteins. This increases the molecular stability in the harsh extracellular environment, and also functions to confer proteolytic resistance (since protein export is a costly process, minimizing its necessity is advantageous). Intracellularly, disulfide bridges between cysteines within a polypeptide support the protein's secondary structure. Insulin is an example of a protein with cystine crosslinking, where two separate peptide chains are connected by a pair of disulfide bonds.
Protein Disulfide Isomerases catalyze the proper formation of disulfide bonds; the cell transfers dehydroascorbic acid to the endoplasmic reticulum which oxidises the environment. In this environment, cysteines are generally oxidized to cystine and no longer functions as a nucleophile.
Dietary sources
Cysteine can be found in meat, red peppers, garlic, onions, broccoli, brussel sprouts, oats, milk, whey protein, and wheat germ. However, it is not classified as an essential amino acid, and can usually be synthesized by the human body under normal physiological conditions if a sufficient quantity of methionine is available.
Production
It is interesting to note that currently the cheapest source of material from which food grade L-cysteine may be purified in high yield is by hydrolysis of molecules in human hair. Other sources include feathers and pig bristles. The companies producing cysteine by hydrolysis are located mainly in China. There is some debate whether or not consuming L-cysteine derived from human hair constitutes cannibalism. Although many other amino acids were accessible via fermentation for some years, L-Cysteine was unavailable until 2001 when a German company introduced a production route via fermentation (non-human, non-animal origin.)
A source of bonded cysteine (cystine) is undenatured bovine whey protein; this is the same form as that in human breast milk.
Applications
Cysteine (mostly in the naturally occurring form L-cysteine) is used for applications in the food, pharmaceutical and personal care industries. One of the largest applications is the production of various flavors. For example, reacting cysteine with sugars in a Maillard reaction yields meat flavors. L-cysteine is also used as a processing aid for baking. Small quantities (in the tens of ppm range) help to soften the dough and thus reduce processing time.
The cysteine derivative N-acetyl cysteine (NAC) is often used as a cough medicine as it breaks up the disulfide bonds in the mucus and thus liquefies it, making it easier to cough up. NAC is also used as a dietary supplement as already indicated above.
In the field of personal care, cysteine is used for permanent wave applications predominantly in Asia. Again the cysteine is used for breaking up the disulfide bonds in the hair's keratin.
Cysteine is a very popular target for site-directed labeling experiments to investigate biomolecular structure and dynamics. Maleimides will selectively attach to cysteine using a covalent michael-addition. Site-directed spin labeling for EPR also uses cysteine extensively.
In a 1994 report released by five top cigarette companies, cysteine is one of the 599 additives to cigarettes. Its use or purpose, however, is unknown, like most cigarette additives.[4] Its inclusion in cigarettes could offer two benefits: Acting as an expectorant, since smoking increases mucus production in the lungs; and increasing the beneficial antioxidant glutathione (which is diminished in smokers).
Sheep
Cysteine is required by sheep in order to produce wool, however it is an essential amino-acid that cannot be synthesised by the sheep and must be taken in as food from grass. This means that during drought conditions sheep stop producing wool; however, transgenic sheep have been developed which can make their own cysteine. a little concentration of cysteine is used to break the disulfide bonds during the solublisation of recombinant protein preparation.
Hangover Remedy
Cysteine has been linked to aiding in the remedy of certain hangover symptoms. It directly counteracts the poisonous effects of acetaldehyde, a particularly toxic by-product of alcohol in the human body. Cysteine attracts the toxin, breaking it down into the non-toxic acetate, a substance similar to vinegar. The actual effectiveness of consuming cysteine as part of a hangover remedy is unclear.[5]
See also
- selenocysteine
- amino acids
- thiols
- cysteine metabolism
ReferencesISBN links support NWE through referral fees
- ↑ Bulaj G, Kortemme T, Goldenberg D (1998). Ionization-reactivity relationships for cysteine thiols in polypeptides.. Biochemistry 37 (25): 8965-72. PMID 9636038.
- ↑ Baker D, Czarnecki-Maulden G (1987). Pharmacologic role of cysteine in ameliorating or exacerbating mineral toxicities.. J Nutr 117 (6): 1003-10. PMID 3298579.
- ↑ 3.0 3.1 (1)
- ↑ http://quitsmoking.about.com/cs/nicotineinhaler/a/cigingredients.htm
- ↑ http://www.lef.org/protocols/prtcl-004.shtml
External links
- Computational Chemistry Wiki
- International Kidney Stone Institute
- International Cystinuria Foundation
- http://www.chemie.fu-berlin.de/chemistry/bio/aminoacid/cystein en.html
- On the hydrophobic nature of cysteine.
- Cystine Kidney Stones
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
