Guinea worm disease
| Guinea worm disease (Dracunculiasis) | |
 A method used to extract a guinea worm from the leg of a human | |
| ICD-10 | B72 |
|---|---|
| ICD-9 | 125.7 |
| DiseasesDB | 3945 |
| eMedicine | ped/616 |
| MeSH | D004320 |
Guinea worm disease (GWD), also called dracunculiasis, is a parasitic infection caused by the nematode (roundworm) Dracunculus medinensis (guinea worm). The disease is spread by drinking water contaminated by copepods that harbor the guinea worm larvae. The larvae mate in the human body and the females mature and develop into two-to-three-foot-long worms, which often forms a painful lesion on a lower limb and may exit excruciatingly through a leg or foot, among other parts of the human body. GWD is the only human disease known to be contracted exclusively by drinking water.
The guinea worm is one of the best historically documented human parasites, even noted in the ancient Ebers Papyrus. Once widely spread through tropical Africa and Asia, GWD was reported in only four countries in 2012 (Chad, Ethiopia, Mali, and South Sudan); Asia is free of the disease. The incidence of the disease has gone from an estimate of more than 3 million cases a year in 1986 to only 542 reported cases in 2012. It may become the second human disease to be eradicated, after smallpox, and the first parasitic disease eradicated.
The endeavor to tackle guinea worm disease reflects a remarkably successful international effort, with over 99% reduction in the disease. And this has been accomplished not with vaccines or medical treatment, but largely through education and behavior change, along with treatment of contaminated water with larvicides and provision of clean water. This successful effort against a painful and deadly disease that spanned two continents provides great inspiration for tackling of the many scourges afflicting humanity.
Overview
Guinea worm disease is a nodular dermatosis produced by the development of Dracunculus parasite in the subcutaneous tissue of mammals. Dracunculus medinensis has been reported in humans, dogs, cats, horses, cattle, and other animals in Africa and Asia, although James Hughes, professor of medicine and public health at Emory University, states that humans are the only reservoir of the disease (McKeever 2013; Nelson 2012). A similar species of the Dracunculus genus, D. insignis, is a parasite which causes Dracunculiasis in dogs, raccoons, minks, foxes, otters, and skunks of North America (McKeever 2013).
The parasite enters a host by way of host ingestion of stagnant water contaminated with copepods (water fleas) infested with guinea worm larvae. The copepods serve as agents of disease transmission. Approximately a year after ingestion, the disease presents with a painful, burning sensation as the female worm forms a blister, usually on the lower limb. The mature worm, now two to three feet in length, often exits the lower limb or foot and is excruciatingly painful (Nelson 2012)
The guinea worm is one of the best historically documented human parasites, including being mentioned in the Egyptian medical Ebers Papyrus, dating from about 1550 B.C.E. (Palmer and Reeder 2005). Tales of the behavior of the guinea worm can also be found in writings from the 2nd century B.C.E., in accounts penned by Greek chroniclers (Piper 2007). The 2nd century B.C.E., Greek writer Agatharchides, described this affliction as being endemic among certain nomads in what is now Sudan and along the Red Sea (Palmer and Reeder 2005). Guinea worm has been found in calcified Egyptian mummies (Carter Center 2013a) and it has been speculated that an Old Testament description of "fiery serpents" may have been referring to guinea worm: "And the Lord sent fiery serpents among the people, and they bit the people; and much people of Israel died" (Numbers 21:4–9) (Palmer and Reeder 2005). The traditional (and still current) method of extracting guinea worm by twisting the worm around a stick may have inspired the rod of Asclepius, a symbol of medicine since Ancient Greek times, which portrays a snake winding around a staff (Blayney 2005).
The name dracunculiasis is derived from the Latin "affliction with little dragons" (Barry 2007), while the common name "guinea worm" appeared after Europeans saw the disease on the Guinea coast of West Africa in the 17th century (Palmer and Reeder 2005). The unusually high incidence of dracunculiasis in the city of Medina led to it being included in part of the disease's scientific name medinensis. Guinea worm is no longer endemic in either location.
Once prevalent in 21 nations in Asia and Africa, with estimates of 3.5 million cases a year in 1986, the disease remains endemic among humans in only four countries in Africa (Carter Center 2013a; Nelson 2012). In 2012, only 542 cases were reported (Carter Center 2013a).
The Carter Center, which has spearheaded the eradication effort with such partners as the World Health Organization and the Centers for Disease Control, has predicted that guinea worm disease "will be the first parasitic disease to be eradicated and the first disease to be eradicated without the use of vaccines or medical treatment" (Carter Center 2013a; Nelson 2012). Former U.S. President Jimmy Carter has been quoted as saying "We are approaching the demise of the last guinea worm who will ever live on earth" (Nelson 2012).
The primary mode of prevention is through behavior change, alongside the provision of clean water sources and the treatment of contaminated drinking water with larvicides. There is no animal or environmental reservoir of D. medinensis and thus the parasite must pass through a host each year to survive (Carter Center 2013a).
Guinea worm and life cycle
| Dracunculus medinensis | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||||||
| Scientific classification | ||||||||||||||||
| ||||||||||||||||
| Dracunculus medinensis (Linnaeus, 1758) | ||||||||||||||||
|
Gordius medinensis Linnaeus, 1758 |
Dracunculus medinensis is a long and very thin nematode (roundworm) (Andrews and Berger 2006). It is the female guinea worm that causes the symptoms of dracunculiasis (Bimi 20007). The adult female is primarily larger than the adult male. The male guinea worm is typically 12 to 29 mm (0.5 to 1.1 inches) while the female can grow to 0.6 to 0.9 meters (2 to 3 feet) long and be as thick as a spaghetti noodle (Palmer and Reeder 2005). The longest adult female recorded was 800 mm (31 in), while the adult male was only 40 mm (1.6 in) (Schmidt and Roberts 2009). The female guinea worm is among the longest nematodes infecting humans (Saleem and Ahmed 2006).
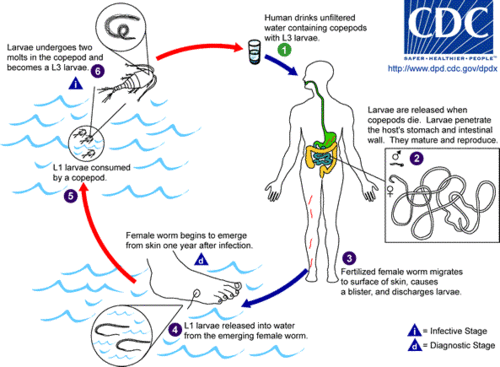
L1 stage larvae are consumed by water fleas ((microscopic arthropods known as copepods) in contaminated drinking water. The larvae develop for approximately two weeks inside the copepods, undergoing two molts and becoming L3 stage larvae. At this stage the larvae can cause guinea worm disease if the infected copepods are not filtered from drinking water and are consumed by humans.
Once inside the body, stomach acid digests and kills the water flea, but not the guinea worm larvae that are sheltered inside. The stage 3 larvae then penetrate the host's stomach or intestinal wall, and enter into the abdominal cavity and retroperitoneal space. After maturing, the male and female worms mate. This takes place approximately three months after infection. The male worm dies after mating and is absorbed (Palmer and Reeder 2005). The female, which contains larvae, burrows into the deeper connective tissues or adjacent to long bones or joints of the extremities (Palmer and Reeder 2005).
Approximately one year after mating, the fertilized females migrate in the subcutaneous tissues towards the surface of the skin, causing formation of painful, ulcerating blisters on the skin, generally on the distal lower extremity (foot)(CDC 2007). Within 72 hours the blister ruptures, exposing one end of the emergent worm. The females can also emerge from other parts of the body, such as the head, torso, upper extremities, buttocks, and genitalia (Schmidt and Roberts 2009). The blister caused by a female guinea worm results in a very painful burning sensation as the worm emerges. The patients then often seek to relieve the burning sensation by placing their foot in water, but when the lesion comes into contact with water, the female worm emerges and releases hundreds of thousands of her stage 1 larvae, contaminating the water supply.
During the next few days, the female worm is capable of releasing more larvae whenever it comes in contact with water as it extends its posterior end through the hole in the host's skin. These larvae contaminate the water supply and are eaten by copepods, then humans, thereby repeating the life-cycle of the disease. Infected copepods can live in the water for only two to three weeks if they are not ingested by a person.
Infection does not create immunity, so people can repeatedly experience guinea worm disease throughout their lives (CDC 2007).
In drier areas just below the Sahara desert, cases of the disease often emerge during the rainy season, which for many agricultural communities is also the planting or harvesting season. Elsewhere, the emerging worms are more prevalent during the dry season, when ponds and lakes are smaller and copepods are thus more concentrated in them. Guinea worm disease outbreaks can cause serious disruption to local food supplies and school attendance (CDC 2007).
Signs and symptoms
As the worm moves downwards, usually to the lower leg, through the subcutaneous tissues, it leads to intense pain localized to its path of travel. The painful, burning sensation experienced by infected people has led to the disease being called "the fiery serpent." Other symptoms include fever, nausea, and vomiting (WHO 2010a).
Treatment
There is no vaccine or medicine to treat or prevent Guinea worm disease (WHO 2010a). Once a Guinea worm begins emerging, the first step is to do a controlled submersion of the affected area in a bucket of water. This causes the worm to discharge many of its larva, making it less infectious. The water is then discarded on the ground far away from any water source. Submersion results in subjective relief of the burning sensation and makes subsequent extraction of the worm easier.
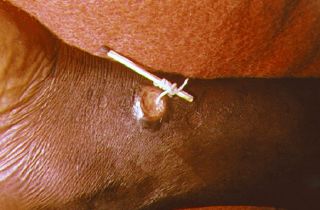
To extract the worm, a person must wrap the live worm around a piece of gauze or a stick. The process can be long, taking anywhere from hours to months. Gently massaging the area around the blister can help loosen the worm up a bit (Carter Center 2013a). This is nearly the same treatment that is noted in the famous ancient Egyptian medical text, the Ebers papyrus from 1550 B.C.E. (Palmer and Reeder 2005). Some people have said that extracting a guinea worm feels like the afflicted area is on fire (WHO 2007; McNeil 2006). However, if the infection is identified before an ulcer forms, the worm can also be surgically removed by a trained doctor in a medical facility.
Although guinea worm disease is usually not fatal, the wound where the worm emerges could develop a secondary bacterial infection such as tetanus, which may be life-threatening—a concern in endemic areas where there is typically limited or no access to health care (ITFDE 1993). Analgesics can be used to help reduce swelling and pain and antibiotic ointments can help prevent secondary infections at the wound site (CDC 2012). however, at least in the Northern region of Ghana, the guinea worm team found that antibiotic ointment on the wound site caused the wound to heal too well and too quickly making it more difficult to extract the worm and more likely that pulling would break the worm. The local team preferred to use something called "Tamale oil" (after the regional capital), which lubricated the worm and aided its extraction. As a practical matter, many patients were also given prophylactic oral antibiotics.
It is of great importance not to break the worm when pulling it out. Broken worms have a tendency to putrefy or petrify. Putrefaction leads to the skin sloughing off around the worm. Petrification is a problem if the worm is in a joint or wrapped around a vein or other important area.
Use of metronidazole or thiabendazole may make extraction easier, but also may lead to migration to other parts of the body (Dhawan 2011).
Prevention
Guinea worm disease can be transmitted only by drinking contaminated water, and can be completely prevented through two relatively simple measures (WHO 2010a):
1. Preventing people from drinking copepod-contaminated water. The cyclops (copepods belonging to the order Cyclopoida) can be seen in clear water as swimming white specks.
- Drinking water drawn only from sources that are free from contamination, such as a borehole or wells.
- Filtering drinking water, using a fine-mesh cloth filter like nylon, to remove the guinea worm-containing crustaceans. Even folding regular cotton cloth over a few times is an effective filter.
- Filtration through ceramic or sand filters
- Boiling
- Developing new sources of drinking water that lack the parasites, or repairing dysfunctional ones.
- Treating water sources with larvicides to kill the copepods (Hopkins et al. 2008).
2. Preventing people with emerging guinea worms from entering water sources used for drinking.
- Community-level case detection and containment is key. This requires staff going door to door looking for cases and a population willing to help and not hide their cases.
- Controlled immersion of emerging worms in buckets of water to reduce the number of larva in the individual worms, followed by discarding the water on dry ground.
- Discouraging all members of the community from setting foot in the drinking water source
- Employing guards at local water sources to prevent people with emerging worms from entering
Epidemiology
In 1986, there were an estimated 3.5 million cases of Guinea worm in 21 nations in Asia and Africa (Carter Center 2013a). Ghana alone reported 180,000 cases in 1989. The number of cases has since been reduced by more than 99.98% to 542 in 2012 (WHO 2013a)—in the four remaining endemic nations of Africa: South Sudan, Chad, Mali, and Ethiopia. This is the lowest number of cases since the eradication campaign began. As of 2010, however, the WHO predicted it will be "a few years yet" before eradication is achieved, on the basis that it took 6–12 years for the countries that have so far eliminated guinea worm transmission to do so after reporting a similar number of cases to that reported in southern Sudan (now South Sudan) in 2009 (WHO 2010b).
The World Health Organization is the international body that certifies whether a disease has been eliminated from a country or eradicated from the world. Endemic countries must report to the International Commission for the Certification of Dracunculiasis Eradication and document the absence of indigenous cases of Guinea worm disease for at least three consecutive years to be certified as Guinea worm-free by the World Health Organization (CDC 2000). The Carter Center also reports the status of the guinea worm eradication program by country (Carter Center 2013b).
By 2007, Benin, Burkina Faso, Chad, Côte d'Ivoire, Kenya, Mauritania, Togo, and Uganda had stopped transmission, and Cameroon, CAR, India, Pakistan, Senegal, Yemen were WHO certified (Carter Center 2013b). At the end of 2012, South Sudan, Mali, Ethiopia, and Chad still had endemic transmission. The major focus is South Sudan (independent after 2011, formerly the southern region of Sudan), which reported 96% of all cases in 2012 (WHO 2013b).
History of the eradication program
The global campaign to eradicate guinea worm disease began at the U.S. Centers for Disease Control and Prevention (CDC) in 1980. In 1986, former U.S. President Jimmy Carter and his not-for-profit organization, The Carter Center, began leading the global campaign, in conjunction with CDC, UNICEF, and WHO (Carter Center 2013c). At this time India, Pakistan, Yemen, and 17 countries in Africa were endemic for this disease and reported a total of 3.5 million cases per year.
Since humans are the principal host for guinea worm, and there is no evidence that D. medinensis has ever been reintroduced to humans in any formerly endemic country as the result of non-human infections, the disease can be controlled by identifying all cases and modifying human behavior to prevent it from recurring (Carter Center 2013a; Bimi et al. 2005). Once all human cases are eliminated, the disease cycle will be broken, resulting in its eradication.
In 1991, the World Health Assembly (WHA) agreed that guinea worm disease should be eradicated (ITFDE 1993). At this time there were 400,000 cases reported each year. The Carter Center has continued to lead the eradication efforts, primarily through its guinea worm eradication program (Carter Center 2006). While other major actors in the eradication of guinea worm disease include WHO, CDC, the Bill & Melinda Gates Foundation, and UNICEF, but the global coalition now includes dozens of other donors, nongovernmental organizations, and institutions, most especially the ministries of health of the affected countries themselves.
In December 2008, the Carter Center announced new financial support totaling US$55 million from the Bill & Melinda Gates Foundation and the United Kingdom Department for International Development (Carter Center 2008). The funds were to help address the higher cost of identifying and reporting the last cases of Guinea worm disease.
On January 30, 2012, the WHO meeting at the Royal College of Physicians in London launched the most ambitious and largest coalition health project ever, known as London Declaration on Neglected Tropical Diseases which aims to end/control dracunculiasis by 2020, among other diseases. This project was declared under the official support of all major pharmaceutical companies, the Bill & Melinda Gates Foundation, the governments of the United States, United Kingdom DFID, United Arab Emirates, and the World Bank (WHO 2012).
Barriers
The eradication of guinea worm disease has faced several challenges:
- Inadequate security in some endemic countries
- Lack of political will from the leaders of some of the countries in which the disease is endemic
- The need for change in behavior in the absence of a magic bullet treatment like a vaccine or medication
- Inadequate funding at certain times (Barry 2007)
One of the most significant challenges facing guinea worm eradication was the Second Sudanese Civil War in southern Sudan, which made southern Sudan largely inaccessible to health workers due to violence (Barry 2007; Carter Center 2013d). To address some of the humanitarian needs in southern Sudan, in 1995 the longest ceasefire in the history of the war was achieved through negotiations by Jimmy Carter (Barry 2007; Carter Center 2013d). Commonly called the "guinea worm cease-fire," both warring parties agreed to halt hostilities for nearly six months to allow public health officials to begin guinea worm eradication programming, among other interventions (Carter Center 2013d; Hopkins and Withers 2002).
Public health officials cite the formal end of the war in 2005 as a turning point in guinea worm eradication because it has allowed health care workers greater access to southern Sudan's endemic areas.
One of the greater recurring challenges in eradicating dracunculiasis has been in South Sudan (formerly southern Sudan), particularly political uncertainty in the country with national elections in 2009 and the referendum on the status of southern Sudan in 2011 resulting in South Sudan's independence. Sporadic insecurity or widespread civil conflict could at any time ignite, thwarting eradication efforts (Hopkins et al. 2008). The remaining endemic communities in South Sudan are remote, poor, and devoid of infrastructure, presenting significant hurdles for effective delivery of interventions against disease. Moreover, residents in these communities are nomadic, moving seasonally with cattle in pursuit of water and pasture, making it very difficult to know where and when transmission occurred. The peak transmission season coincides with the rainy season, hampering travel by public health workers (WHO 2008).
ReferencesISBN links support NWE through referral fees
- Barry, M. 2007. The tail end of guinea worm—global eradication without a drug or a vaccine. N. Engl. J. Med. 356(25): 2561–4. PMID 17582064. Retrieved August 1, 2013.
- Bimi, L. 2007. Potential vector species of Guinea worm (Dracunculus medinensis) in Northern Ghana. Vector-Borne and Zoonotic Diseases 7(3): 324–329. PMID 17767406.
- Bimi, L., A. R. Freeman, M. L. Eberhard, E.Ruiz-Tiben, and N. J. Pieniazek. 2005. Differentiating Dracunculus medinensis from D. insignis, by the sequence analysis of the 18S rRNA gene. Annals of Tropical Medicine & Parasitology 99(5): 511–517. Retrieved August 1, 2013.
- Blayney, K. 2005. The caduceus vs the staff of Asclepius ((Asklepian). drblayney.com. Retrieved August 1, 2013.
- Carter Center. 2006. 2006 Gates Award for Global Health: The Carter Center. Carter Center. Retrieved July 31, 2013.
- Carter Center. 2008. Guinea worm cases hit all-time low: Carter Center, WHO, Gates Foundation, and U.K. Government commit $55 million toward ultimate eradication goal. Carter Center. Retrieved July 31, 2013.
- Carter Center. 2013a. Guinea Worm Eradication Program. Carter Center.
- Carter Center. 2013b. Activities by country: Guinea Worm Eradication Program. Carter Center.
- Carter Center. 2013c. International Task Force for Disease Eradication—Original Members (1989–1992). Carter Center. Retrieved July 30, 2013.
- Carter Center. 2013d. The Republic of Sudan and The Republic of South Sudan. Carter Center. Retrieved July 31, 2013.
- Centers for Disease Control and Prevention (CDC). 2000. Progress toward global dracunculiasis eradication, June 2000. JAMA 284(14): 1778-1779. PMID 11041744. Retrieved August 1, 2013.
- Centers for Disease Control and Prevention (CDC). 2007. Progress toward global eradication of dracunculiasis, January 2005-May 2007. MMWR Morb. Mortal. Wkly. Rep. 56(32): 813–7. PMID 17703170. Retrieved August 1, 2013.
- Centers for Disease Control and Prevention (CDC). 2012. Parasites: Dracunculiasis (also known as guinea worm disease). CDC. Retrieved July 29, 2013.
- Centers for Disease Control and Prevention (CDC). 2013. Dracunculus medinensis. CDC. Retrieved August 1, 2013.
- Dhawan, V. K. 2011. Dracunculiasis treatment & management. MEDSCAPE. Retrieved August 1, 2013.
- Hopkins, D. R., and P. C. Withers. 2002. Sudan's war and eradication of dracunculiasis. Lancet 360: s21–2.
- Hopkins, D. R. , F. Richards, E. Ruiz-Tiben, P. Emerson, and P. Withers. 2008. Dracunculiasis, onchocerciasis, schistosomiasis, and trachoma. Annals of the New York Academy of Sciences 1136: 45–52. PMID 17954680.
- Hopkins, D. R., E. Ruiz-Tiben, P. Downs, P. C. Withers, and J. H. Maguire. 2005. Dracunculiasis eradication: The final inch. Am J. Trop Med. Hyg 73(4): 669-675. PMID 16222007. Retrieved August 1, 2013.
- Hopkins, D.R., E. Ruiz-Tiben, P. Downs, P. C. Withers, and S. Roy. 2008. Dracunculiasis eradication: Neglected no longer. Am. J. Trop. Med. Hyg. 79(4): 474–9. PMID 18840732. Retrieved August 1, 2013.
- International Task Force for Disease Eradication (ITFDE). 1993. Recommendations of the International Task Force for Disease Eradication. MMWR Recomm Rep 42(RR–16): 1–25. PMID 8145708. Retrieved August 1, 2013.
- James, W. D. and T. G. Berger. 2006. Andrews' Diseases of the Skin: Clinical Dermatology. Saunders Elsevier. ISBN 0721629210.
- McKeever Dermatology Clinics. 2013. Dracunculiasis. Mckeever Dermatology Clinics. Retrieved July 24, 2013.
- McNeil, D. G. 2006. Dose of tenacity wears down a horrific disease. New York Times March 26, 2006. Retrieved July 29, 2013.
- Nelson, R. 2012. The last worm: A dreaded tropical disease is on the verge of eradication. Scientific American 302(1):24.
- Palmer, P. E. S., and M. M. Reeder. 2005. Guinea worm infection (Dracunculiasis). In Palmer and Reeder's, The Imaging of Tropical Disease (DVD, Internet version)]. Reproduced from the Original Text by The Uniformed Services University of the Health Sciences, and Distributed by The American College of Radiology, the RSNA, the Radiology Outreach Foundation, and The International Society of Radiology With the Kind Permission of Springer Publishing, the Authors, and the Above Institutions. Retrieved July 24, 2013.
- Piper, R. 2007. Extraordinary Animals: An Encyclopedia of Curious and Unusual Animals. Greenwood Press. ISBN 9780313339226.
- Saleem, T. B., and I. Ahmed. 2006. "Serpent" in the breast. Journal of Ayub Medical College Abbottabad 18(4): 67–68. Retrieved August 1, 2013.
- Schmidt, G. D., and L. S. Roberts. 2009. Foundations of Parasitology, 8th edition. McGraw-Hill. ISBN 9780071284585.
- World Health Organization (WHO). 2007. World moves closer to eradicating ancient worm disease. World Health Organization. Retrieved July 29, 2013.
- World Health Organization (WHO). 2008. Dracunculiasis eradication. MMWR Morb. Mortal. Wkly. Rep. 83(18): 159-167. PMID 18453066. Retrieved August 1, 2013.
- World Health Organization (WHO). 2009. WHO certifies seven more countries as free of guinea-worm disease. World Health Organization. Retrieved August 1, 2013.
- World Health Organization (WHO). 2010a. Dracunculiasis. World Health Organization. Retrieved July 25, 2013.
- World Health Organization (WHO). 2010b. Dracunculiasis eradication: Global surveillance summary, 2009. Weekly epidemiological record 85: 165–176. Retrieved July 31, 2013.
- World Health Organization (WHO). 2012. WHO roadmap inspires unprecedented support to defeat neglected tropical diseases. World Health Organization. Retrieved August 1, 2013.
- World Health Organization Collaborating Center for Research, Training and Eradication of Dracunculiasis (WHO). 2013a. CDC Guinea Worm Wrap-Up #216 Public Health Service Centers for Disease Control and Prevention (CDC). Retrieved July 26, 2013.
- World Health Organization Collaborating Center for Research, Training and Eradication of Dracunculiasis (WHO). 2013b. CDC Guinea Worm Wrap-Up #217. Public Health Service Centers for Disease Control and Prevention (CDC). Retrieved July 27, 2013.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.