Cocaine
| |
| |
Cocaine
| |
| Systematic name | |
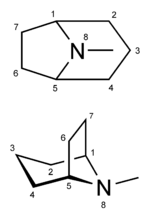
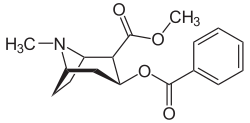
| IUPAC name methyl (1R,2R,3S,5S)-3- (benzoyloxy)-8-methyl-8-azabicyclo[3.2.1] octane-2-carboxylate | |
| Identifiers | |
| CAS number | 50-36-2 |
| ATC code | N01BC01 R02AD03, S01HA01, S02DA02 |
| PubChem | 5760 |
| DrugBank | DB00907 |
| Chemical data | |
| Formula | C17H21NO4Â |
| Mol. weight | 303.353Â g/mol |
| SMILES | CN1[C@H]2CC[C@@H]1[C@H]([C@H](C2)OC(=O)c3ccccc3)C(=O)OC |
| Synonyms | methylbenzoylecgonine, benzoylmethylecgonine, ecgonine methyl ester benzoate, 2b-Carbomethoxy â3b-benzoyloxy tropane |
| Physical data | |
| Melt. point | 98°C (208°F) |
| Boiling point | 187°C (369°F) |
| Solubility in water | HCl: 1800â2500 mg/mL (20°C) |
| Pharmacokinetic data | |
| Bioavailability | Oral: 33%[1] Insufflated: 60[2]â80%[3] Nasal Spray: 25[4]â43%[1] |
| Metabolism | Hepatic CYP3A4 |
| Half life | 1 hour |
| Excretion | Renal (benzoylecgonine and ecgonine methyl ester) |
| Therapeutic considerations | |
| Pregnancy cat. | C |
| Legal status | ? |
| Dependence Liability | High |
| Routes | Topical, Oral, Insufflation, IV, PO |
Cocaine is a crystalline tropane alkaloid (benzoylmethylecgonine, C17H21NO4) found in the leaves of the coca plant and best known in its concentrated form as an addictive, and generally illegal, psychoactive recreational drug.
Although the amount of cocaine in coca leaves is low, when this alkaloid is chemically extracted and concentrated it results in a powerful nervous system stimulant, which is generally used nasally, smoked, or injected. As such, cocaine can be highly addictive and have deleterious impacts on the brain, heart, respiratory system, kidneys, sexual system, and gastrointestinal tract. In most countries, the production, distribution, sale, and possession of cocaine products is restricted and/or illegal. However, cocaine also has some medical use and in some countries is available by prescription for such purposes as external application to the skin to numb pain, although derivatives such as lidocaine and novocaine have largely replaced it.
Use of concentrated cocaine yields pleasure through its interference with neurotransmitters of the sympathetic nervous system, such as blocking dopamine from being reabsorbed and thus resulting in continual stimulation. As such, cocaine subverts a natural system for experiencing pleasure and, ironically, the user can reach a state in which he or she has difficulty experiencing pleasure without the drug. In addition to medical problems from the drug, including sudden death, cocaine is one of the most addictive recreational drugs and intense cravings can be created even after one use. The use of cocaine can create a tolerance, requiring an increasing dose for stimulation.
There is a huge worldwide market for cocaine. The United Nations Office of Drugs and Crime estimated that in 2009 the US cocaine market was $37 billion and the West and Central European cocaine market was US$ 33 billion.
For the plant, cocaine seems to serve a valuable function as an effective insecticide, limiting damage from herbivorous insects.
Overview
Cocaine is a tropane alkaloid. Tropane alkaloids are a class of alkaloids (naturally occurring chemical compounds that contain mostly basic nitrogen atoms) and secondary metabolites in which the chemical structure includes a tropane ring (nitrogenous bicyclic organic structure). Well-known alkaloids include caffeine, nicotine, morphine, theobromine, mescaline, strychnine, quinine, and codeine. Well-known tropane alkaloids, in addition to cocaine, include atropine and ecgonine (a precursor and metabolite of cocaine). Cocaine has the chemical formula C17H21NO4 and is also known as benzoylmethylecgonine or methyl benzoyl ecgonine.
Cocaine is found in coca plants, which are indigenous to South America. There are four varieties of these tropical plants that are cultivated: Erythroxylum coca var. coca (Bolivian or HuĂĄnuco coca), E. coca var. ipadu (Amazonian coca), E. novogranatense var. novogranatense (Colombian coca), and E. novogranatense var. truxillense (Trujillo coca). The name cocaine comes from the name of the coca plant plus the alkaloid suffix -ine.
Cocaine is the most concentrated of the dozen or more alkaloids that have been identified in the coca plant. Concentrations vary by variety and region, but leaves have been reported as having between 0.25% and 0.77% (Plowman and Rivier 1983), between 0.35% and 0.72% by dry weight (Nathanson et al. 1993), and between 0.3% and 1.5% and averaging 0.8% in fresh leaves (Casale and Klein 1993). In unprocessed form, coca leaves have been used for thousands of years in South America for various religious, social, medicinal, and nutritional purposes, including to control hunger and combat the impacts of high altitudes. However, since the alkaloid cocaine is present in only trace amounts in the leaves, it does not cause the euphoric and psychoactive effects associated with use of the drug.
When processed and concentrated by chemical extraction from large quantities of coca leaves, cocaine is a powerful stimulant. The extract from the leaves is hydrolysed and esterified with methanol and benzoic acid to produce the hydrochloride salt of cocaine.
Biologically, cocaine acts as a serotoninânorepinephrineâdopamine reuptake inhibitor, also known as a triple reuptake inhibitor (TRI). For example, Marieb and Hoehn (2010) note the impact of cocaine hooking up to the dopamine reuptake transporter protein, thus blocking reabsorption of dopamine. With this neurotransmitter remaining in the synapse, the post-synaptic receptor cells are stimulated again and again, allowing the body to experience over and over this reward system and associated high, along with increased heart rate, sexual appetite, and blood pressure. However, as a result, the system releases less and less dopamine and the reward system goes dry, and the cocaine user, in addition to becoming anxious, find himself or herself "in a very real sense, unable to experience pleasure without the drug." However, more cocaine just suppresses dopamine release even more.
Unlike most molecules, cocaine has pockets with both high hydrophilic and lipophilic efficiency, violating the rule of hydrophilic-lipophilic balance. This causes it to cross the bloodâbrain barrier far better than other psychoactive chemicals and may even induce blood-brain barrier breakdown (Sharma et al. 2009; Dietrich 2009). Marieb and Hoehn (2010) note that one way that might be developed to tackle addiction would be to prompt the immune system to bind cocaine molecules and prevent them from entering the brain.
Cocaine is best known worldwide for its illegal use as a recreational drug. This concentrated form of cocaine is used nasally (nasal insufflation is also known as "snorting," "sniffing," or "blowing" and involves absorption through the mucous membranes lining the sinuses), injected (the method that produces the highest blood levels in the shortest time), or smoked (notably the cheaper, more potent form called "crack"). It may also be administered orally (rubbed on gums). Among forms of cocaine use are cocaine hydrochloride, natural leaf, cocaine paste, or freebase.
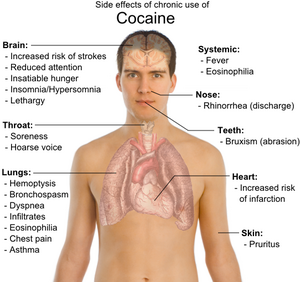
Cocaine use can be highly addictive, causing intense cravings for the drug, and can have deleterious impacts on the brain, heart, respiratory system, kidneys, sexual system, and gastrointestinal tract (WebMD 2013a). For example, it can result in a heart attack or strokes, even in young people,and it can cause ulcers and sudden kidney failure, and it can impair sexual function (WebMD 2013a).
The possession, distribution, and sale of cocaine products is illegal for non-medicinal / non-government sanctioned purposes in virtually all parts of the world. Internationally, it is regulated by the Single Convention on Narcotic Drugs, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. In the United States, the manufacture, importation, possession, and distribution of cocaine is additionally regulated by the 1970 Controlled Substances Act. Cocaine is generally treated as a 'hard drug', with severe penalties for possession and trafficking.
The United Nations Office of Drugs and Crime estimated that in 2009, the US cocaine market was $37 billion (and shrinking over the past ten years) and the West and Central European Cocaine market was US$ 37 billion (and increasing over the past ten years) (USODC 2011).
The coca leaves have been used unprocessed for thousands of years in South America for various religious, social, medicinal, and nutritional purposes, including in the Andean countries to make a herbal tea with mild stimulant effects. However, since the alkaloid cocaine is present in only trace amounts in the leaves, it does not cause the euphoric and psychoactive effects associated with use of the drug. The Coca-Cola company uses a cocaine-free coca extract. In the early days of the manufacture of Coca-Cola beverage, the formulation did contain some cocaine, although within a few years of its introduction it already was only trace amounts. Cocaine is available as a prescription for such purposes as external application to the skin to numb pain.
For the plant, cocaine is believed to serve as a naturally occurring insecticide, with the alkaloid exerting such effects at concentrations normally found in the leaves (Nathanson et. al. 1993). It has been observed that compared to other tropical plants, coca seems to be relatively pest free, with little observed damage to the leaves and rare observations of herbivorous insects on plants in the field (Nathanson et al. 1993).
Medical effects
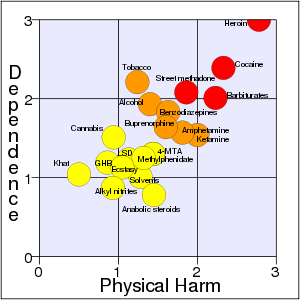
Cocaine acts in the brain on areas that acts to reward people with pleasure for behaviors important to survival individually and as a species, such as food, sex, and healthy pleasure (Marieb and Hoehn 2010; WebMD 2013a; Spanage and Weiss 1999). Involving the brain neurotransmitters in this area, it is a powerful nervous system stimulant (WHO 2004). Its effects can last from 15â30Â minutes to an hour or two, depending on dosage and the route of administration (WHO 2007; WebMD 2013a). However, it can have serious negative effects on the heart, brain, lungs, and emotions, including the danger of sudden death (WebMD). It was ranked the second most addictive and harmful recreational drug (of 20 studied) by Nutt et al. (2007), exceeded only by heroin.
On the one hand, users of cocaine report a euphoria (feeling "high"), with an increased sense of alertness, feelings of well-being, competence, and "supremacy," enhanced energy and motor activity, and sexuality (WebMD 2013a).
On the other hand, some users report the high as also being accompanied with anxiety, irritability, paranoia, and restlessness, particularly during the comedown (WebMd 2013a). With excessive dosage or prolonged use, itching, tachycardia, tremors, convulsions, hallucinations, and paranoid delusions can result (WHO 2004; Zhao 2008). Overdoses cause hyperthermia (elevated body temperature) and a marked elevation of blood pressure. Cocaine constricts blood vessels, dilates the pupils, and increases heart rate and blood pressure.
In terms of the circulatory system, the increase of heart rate and blood pressure, while restricting arteries supplying blood, can result in a heart attack, even in youth without heart disease (WebMD 2013a). An abnormal heart rhythm called arrhythmia can be triggered. In terms of the brain, the constriction of blood vessels in the brain can cause strokes, even in young people without other stroke risk factors (WebMD 2013a). Cocaine can double both the risks of hemorrhagic and ischemic strokes (Jeffrey and Vega 2008) and increase the risk of other infarctions, such as myocardial infarction (Vasica and Tennant 2002). Cocaine can cause seizures. Sudden death has been known to occur, such as the case of Len Bias, considered by some as one of the greatest American college basketball athletes, who died two days after being drafted by the Boston Celtics because of a cardiac arrhythmia induced by use of cocaine.
The constriction of blood vessels supplying the gastrointestinal tract can lead to oxygen starvation and the development of ulcers or perforation of the stomach and intestines (WebMD 2013a). Cocaine use can also cause a wide array of kidney diseases and renal failure (Jaffe and Kimmel 2006; van der Woude 2000). Kidney failure can suddenly occur through a process known as rhabdomyolysis (WebMD 2013a).
While sexual appetite may be increased, cocaine use can impair sexual function in men and women, including impaired ejaculation in men (WebMd 2013a).
In terms of the lungs and respiratory system, physical side effects from chronic smoking of cocaine include hemoptysis, bronchospasm, pruritus, fever, diffuse alveolar infiltrates without effusions, pulmonary and systemic eosinophilia, chest pain, lung trauma, sore throat, asthma, hoarse voice, dyspnea (shortness of breath), and an aching, flu-like syndrome. Permanent lung damage can result in some users.
The experience of insatiable hunger, aches, insomnia/oversleeping, lethargy, and persistent runny nose are often described as very unpleasant. Depression with suicidal ideation may develop in very heavy users.
Chronic intranasal usage can degrade the cartilage separating the nostrils (the septum nasi), leading eventually to its complete disappearance. Due to the absorption of the cocaine from cocaine hydrochloride, the remaining hydrochloride forms a dilute hydrochloric acid (Pagliaro and Pagliaro 2004).
Cocaine may also greatly increase this risk of developing rare autoimmune or connective tissue diseases such as lupus, Goodpasture's disease, vasculitis, glomerulonephritis, StevensâJohnson syndrome and other diseases (Trozak and Gould 1984; Peces et al. 1999; Moore and Richardson 1998).
Cocaine often is a cause of involuntary tooth grinding, known as bruxism, which can deteriorate tooth enamel and lead to gingivitis (Baigent 2003). Additionally, stimulants like cocaine, methamphetamine, and even caffeine cause dehydration and dry mouth. Since saliva is an important mechanism in maintaining one's oral pH level, chronic stimulant abusers who do not hydrate sufficiently may experience demineralization of their teeth due to the pH of the tooth surface dropping too low (below 5.5).
Chronic cocaine intake causes brain cells to adapt functionally to strong imbalances of transmitter levels in order to compensate extremes. Thus, receptors disappear from the cell surface or reappear on it, resulting more or less in an "off" or "working mode" respectively, or they change their susceptibility for binding partners (ligands)Â â mechanisms called down-/upregulation. Marieb and Hoehn (2010) state that the blocking of dopamine uptake by repeated use of cocaine causes the reward system to effectively go dry, as the system releases less and less dopamine, and "the cocaine user becomes anxious and, in a very real sense, unable to experience pleasure without the drug." As the postsynaptic cells sprout new receptors to pick up the dopamine signals, a vicious cycle begins where cocaine "is needed to experience pleasure, but using it suppresses dopamine release even more" (Marieb and Hoehn 2010). A loss of vesicular monoamine transporters, neurofilament proteins, and other morphological changes appear to indicate a long term damage of dopamine neurons. All these effects contribute a rise in tolerance thus requiring a larger dosage to achieve the same effect (Lowinson et al. 2004). On the other hand, a study by D'Haenen et al. (2002) suggests cocaine abusers do not show normal age-related loss of striatal dopamine transporter (DAT) sites, suggesting cocaine has neuroprotective properties for dopamine neurons.
Cocaine can often cause reduced food intake, many chronic users lose their appetite and can experience severe malnutrition and significant weight loss.
The lack of normal amounts of serotonin and dopamine in the brain is the cause of the dysphoria and depression felt after the initial high.
Cocaine is extensively metabolized, primarily in the liver, with only about 1% excreted unchanged in the urine. The metabolism is dominated by hydrolytic ester cleavage, so the eliminated metabolites consist mostly of benzoylecgonine (BE), the major metabolite, and other significant metabolites in lesser amounts such as ecgonine methyl ester (EME) and ecgonine. Further minor metabolites of cocaine include norcocaine, p-hydroxycocaine, m-hydroxycocaine, p-hydroxybenzoylecgonine (pOHBE), and m-hydroxybenzoylecgonine (Kolbrich et al. 2006).
Cocaine has been held responsible for more visits to US emergency rooms than any other illegal drug (WebMD 2013a). The amount of sudden-deaths from cocaine also is not a rare phenomenon and in one study the cause of cocaine-related sudden death was found to cardiovascular in 62% of the cases, cerebrovascular in 14%, excited delirium in 14%, respiratory in 5%, and and metabolic in 5% (Nainggolan 2010). Drs. Richard Lange and L David Hillis of the University of Texas Health Science Center note "The notion that recreational cocaine use is 'safe' should be dispelled, since even small amounts may have catastrophic consequences" (Nainggolan 2010).
Addiction
Cocaine dependence (or addiction) is psychological dependency on the regular use of cocaine. Cocaine dependency may result in physiological damage, lethargy, psychosis, depression, akathisia, and fatal overdose.
Physical withdrawal is not dangerous. Physiological changes caused by cocaine withdrawal include vivid and unpleasant dreams, insomnia or hypersomnia, increased appetite and psychomotor retardation or agitation (Lowinson et al. 2004).
Among the notable cocaine-related deaths from cocaine have been basketball player Len Bias, baseball player Ken Caminiti, Quiet Riot singer Kevin DuBrow, The Who musician John Entwistle, actor Chris Farley (along with morphine), model Katy French, The Righteous Brothers musician Bobby Hatfield, the Pretenders musician James Honeyman-Scott, Blind Melon singer Shannon Hoon, actress/singer Whitney Houston (drowning under the influence), Grateful Dead musician Brent Mydland, actor River Phoenix (along with heroin), the Temptations musician David Ruffin, baseball player Rod Scurry, and musician Ike Turner.
Mechanism of action
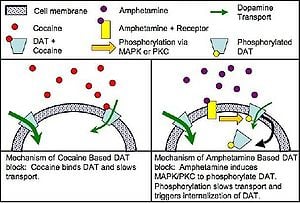
The human brain appears to be hardwired with a reward system that provides pleasure when humans engage in various behaviors that are important to individual or species survival, such as romantic love, sex, and food. Humans ability to feel good involves brain neurotransmitters in this reward system, including dopamine released by neurons in areas known as the ventral tegmental area (VTA), the amygdala, and the nucleus accumbens (Marieb and Hoehn 2010; Spanage and Weiss 1999).
Various drugs of abuse can subvert this reward system, one of which is cocaine. These drugs can cause an addictive pleasure flush by flooding the brain with neurotransmitter-like chemicals or causing a build-up of neurotransmitters such as dopamine. However, this short-lived pleasure also comes with some serious side-effects, including the brain making and releasing less neurotransmitters on its own.
A major effect of cocaine on the central nervous system is the blockade of the dopamine reuptake transporter protein and thus blocking the reabsorption of dopamine. Dopamine transmitter released during neural signaling is normally recycled via the transporter protein; in other words, the transporter binds the transmitter and pumps it out of the synaptic cleft back into the presynaptic neuron, where it is taken up into storage vesicles. By binding tightly with the dopamine transporter, cocaine forms a complex that blocks the transporter's function. The dopamine transporter can no longer perform its reuptake function, and thus dopamine accumulates in the synaptic cleft. This results in an enhanced and prolonged postsynaptic effect of dopaminergic signaling at dopamine receptors on the receiving neuron. In other words, by the dopamine remaining in the synapse, the post-synaptic receptor cells are triggered again and again, allowing a prolonged pleasure flush.
When the dopamine uptake is blocked by repeated use of cocaine, the system reacts by releasing less and less dopamine and "the reward system effectively goes dry" (Marieb and Hoehn 2010). In other words, prolonged exposure to cocaine leads to homeostatic dysregulation of normal dopaminergic signaling via down-regulation of dopamine receptors and enhanced signal transduction. The decreased dopaminergic signaling after chronic cocaine use may contribute to depressive mood disorders and sensitize this important brain reward circuit to the reinforcing effects of cocaine (for example, enhanced dopaminergic signaling only when cocaine is self-administered). This sensitization contributes to the intractable nature of addiction and relapse.
Dopamine-rich brain regions such as the ventral tegmental area, nucleus accumbens, and prefrontal cortex are frequent targets of cocaine addiction research. Of particular interest is the pathway consisting of dopaminergic neurons originating in the ventral tegmental area that terminate in the nucleus accumbens. This projection may function as a "reward center," in that it seems to show activation in response to drugs of abuse like cocaine in addition to natural rewards like food or sex (Spanage and Weiss 1999). While the precise role of dopamine in the subjective experience of reward is highly controversial among neuroscientists, the release of dopamine in the nucleus accumbens is widely considered to be at least partially responsible for cocaine's rewarding effects. This hypothesis is largely based on laboratory data involving rats that are trained to self-administer cocaine. If dopamine antagonists are infused directly into the nucleus accumbens, well-trained rats self-administering cocaine will initially increase responding only to stop completely, thereby indicating that cocaine is no longer reinforcing (i.e. rewarding) the drug-seeking behavior.
Cocaine also effects seratonin (5-hydroxytryptamine, 5-HT), a monoamine neurotransmitter widely thought to be a contributor to feelings of well-being and happiness. Cocaine has been shown to inhibit the re-uptake of 5-HT3. The overabundance of 5-HT3 receptors in cocaine conditioned rats display this trait; however, the exact effect of 5-HT3 in this process is unclear (Carta et al. 2003). The 5-HT2 receptor (particularly the subtypes 5-HT2AR, 5-HT2BR and 5-HT2CR) show influence in the evocation of hyperactivity displayed in cocaine use (Filip et al. 2004).
Sigma receptors are affected by cocaine, as cocaine functions as a sigma ligand agonist (NIH/NIDA 2003). Sigma receptors are proteins found in the brain (and other parts of the body). The impact of cocaine on these sigma receptions may be part of the reason for cocaine's suppression of the immune system (NIH/NIDA 2003). Another specific receptor cocaine has been demonstrated to function on is NMDA (Lluch et al. 2005).
Cocaine also blocks sodium channels, thereby interfering with the propagation of action potentials; thus, like lignocaine and novocaine, it acts as a local anesthetic. It also functions on the binding sites to the dopamine and serotonin sodium dependent transport area as targets as separate mechanisms from its reuptake of those transporters; unique to its local anesthetic value, which makes it in a class of functionality different from both its own derived phenyltropanes analogues (which have that removed) and the amphetamine class of stimulants (which as well altogether lack that). In addition to this cocaine has some target binding to the site of the Kappa-opioid receptor as well. Cocaine also causes vasoconstriction, thus reducing bleeding during minor surgical procedures. The locomotor enhancing properties of cocaine may be attributable to its enhancement of dopaminergic transmission from the substantia nigra.
The impact of the neurotransmitter glutamate is also believed to be important to maintaining addiction, as glutamate signaling seems to cause permanent brain changes that lead to "compulsive drug-seeking behavior elicited by external cues" (Marieb and Hoehn 2010). Mice lacking a particular glutamate receptor are willing to try cocaine but do not become addicted (Marieb and Hoehn 2010). These combined dopamine and glutamate systems are so strong that years later, certain setting can create intense cravings for cocaine (Marieb and Hoehn 2010).
Because nicotine increases the levels of dopamine in the brain, many cocaine users find that consumption of tobacco products during cocaine use enhances the euphoria. This, however, may have undesirable consequences, such as uncontrollable chain smoking during cocaine use (even users who do not normally smoke cigarettes have been known to chain smoke when using cocaine), in addition to the detrimental health effects and the additional strain on the cardiovascular system caused by tobacco.
Forms
Cocaine in its purest form is a white, pearly product. Cocaine appearing in powder form is a salt, typically cocaine hydrochloride. Street market cocaine is frequently adulterated or âcutâ with various powdery fillers to increase its weight; the substances most commonly used in this process are baking soda; sugars, such as lactose, dextrose, inositol, and mannitol; and local anesthetics, such as lidocaine or benzocaine, which mimic or add to cocaine's numbing effect on mucous membranes. Cocaine may also be "cut" with other stimulants such as methamphetamine. Adulterated cocaine is often a white, off-white or pinkish powder.
Salts. Cocaine is a weakly alkaline compound (an "alkaloid") and can therefore combine with acidic compounds to form various salts. The hydrochloride (HCl) salt of cocaine is by far the most commonly encountered, although the sulfate (-SO4) and the nitrate (-NO3) are occasionally seen. Different salts dissolve to a greater or lesser extent in various solvents. The hydrochloride salt is polar in character and is quite soluble in water. Powdered cocaine is commonly known as "coke" or "blow" and users can snort the powder (inhale through the nose) and into the bloodstream, or dissolve in water and inject directly into the bloodstream.
Basic. As the name implies, âfreebaseâ or "free base" is the base form of cocaine, as opposed to the salt form. It is practically insoluble in water whereas hydrochloride salt is water soluble. (Most alkaloids are unstable in their pure form and exist in ionic salt form. The salts usually exhibit greater water solubility. Common counterions include chloride, bromide, acetate and oxalate. Because of the ubiquity of chloride salts, formed from the reaction of the amine with hydrochloric acid, these amine derivatives are known as the hydrochlorides.) Pure cocaine is prepared by neutralizing its compounding salt with an alkaline solution, which will precipitate to non-polar basic cocaine. It is further refined through aqueous-solvent Liquid-liquid extraction.
The term "freebasing" means converting an ionic form into free base. It can refer to deprotonating the hydrochloride salt form of cocaine to free base form. The free base is preferred for smoking. Smoking freebase cocaine has the additional effect of releasing methylecgonidine into the user's system due to the pyrolysis of the substance (a side effect which insufflating or injecting powder cocaine does not create). Some research suggests that smoking freebase cocaine can be even more cardiotoxic than other routes of administration (Scheidweiler et al. 2003; Yang et al. 2001; Fandiño et al. 2002).
Crack cocaine. Crack is a lower purity form of free-base cocaine that is usually produced by neutralization of cocaine hydrochloride with a solution of baking soda (sodium bicarbonate, NaHCO3) and water, producing a very hard/brittle, off-white-to-brown colored, amorphous material that contains sodium carbonate, entrapped water, and other by-products as the main impurities. The color of âcrackâ cocaine depends upon several factors including the origin of the cocaine used, the method of preparationâwith ammonia or baking sodaâand the presence of impurities, but will generally range from white to a yellowish cream to a light brown. Its texture will also depend on the adulterants, origin and processing of the powdered cocaine, and the method of converting the base. It ranges from a crumbly texture, sometimes extremely oily, to a hard, almost crystalline nature.
The "freebase" and "crack" forms of cocaine are usually administered by vaporization of the powdered substance into smoke, which is then inhaled. The origin of the name "crack" comes from the "crackling" sound (and hence the onomatopoeic moniker âcrackâ) that is produced when the cocaine and its impurities (i.e. water, sodium bicarbonate) are heated past the point of vaporization (Nelson 1998). Pure cocaine base/crack can be smoked because it vaporizes smoothly, with little or no decomposition at 98 °C (208 °F) (Miller et al. 2009), which is below the boiling point of water. In contrast, cocaine hydrochloride does not vaporize until heated to a much higher temperature (about 197°C), and considerable decomposition/burning occurs at these high temperatures. This effectively destroys some of the cocaine, and yields a sharp, acrid, and foul-tasting smoke.
Unprocessed coca leaf. Coca leaves have been used unprocessed for thousands of years in South America for various religious, social, medicinal, and nutritional purposes, including to control hunger and combat the impacts of high altitudes. Chewing of unadulterated coca leaves has been a tradition in the Andes for thousands of years and remains practiced by millions in South America today (Cortes 2013). Individuals may suck on wads of the leaves and keep them in their cheeks for hours at a time, often combining with chalk or ask to help dissolve the alkaloids into the saliva (Boucher 1991). Unprocessed coca leaves are also commonly used in the Andean countries to make a herbal tea with mild stimulant effects. However, since the alkaloid cocaine is present in only trace amounts in the leaves, it does not cause the euphoric and psychoactive effects associated with use of the drug. (See the article coca.)
Routes of administration
Cocaine powder can be inhaled through the nose or dissolved in water and injected into the bloodstream, as well as rubbed along the gum line. The freebase form can be smoked. Cocaine can also be applied to the skin as an topical anesthetic. Coca leaf can be chewed and brewed into a tea. Injecting and smoking leads to faster absorption into the bloodstream than snorting and a quicker, stronger high, but faster absorption also tends to mean a shorter duration of the high (5-10 minutes for smoking versus 15-30 minutes for snorting)(Botany Central 2013).
Insufflation ("snorting," "sniffing," or "blowing") involves breathing in the powder through the nose and in that manner absorption into the bloodstream. Prior to insufflation, cocaine powder is divided into very fine particles. Rolled up banknotes, hollowed-out pens, cut straws, and other such items are often used to insufflate cocaine. Upon snorting, the drug coats and is absorbed through the mucous membranes lining the sinuses. Any material not directly absorbed through the mucous membranes is collected in mucus and swallowed. When insufflating cocaine, absorption through the nasal membranes is approximately 30â60%, with higher doses leading to increased absorption efficiency.
Nasal insufflation is the most common method of ingestion of recreational powdered cocaine in the Western world. In a study of cocaine users, the average time taken to reach peak subjective effects was 14.6Â minutes (Volkow et al. 2000). Physiological and psychotropic effects from nasally insufflated cocaine are sustained for approximately 40â60Â minutes after the peak effects are attained (Barnett et al. 1981). Snorting involves slower absorption into the bloodstream; however, as with other means of administration sudden death remains a risk, as with the other medical complications, including potential damage to the inside of the nose due to cocaine highly constricting blood vessels and therefore blood and oxygen/nutrient flow to that area. In addition, a study by Bonkovsky and Mehta (2001) reported that, just like shared needles, the sharing of straws used to "snort" cocaine can spread blood diseases such as hepatitis C.
Injection. Injection, involving administering the drug directly to the bloodstream by the use of needles, provides the highest blood levels of drug in the shortest amount of time. Volkow et al. (2000) found that the average time taken to reach peak subjective effects was 3.1Â minutes. The euphoria passes quickly. Aside from the toxic effects of cocaine, there is also danger of circulatory emboli from the insoluble substances that may be used to cut the drug. Subjective effects not commonly shared with other methods of administration include a ringing in the ears moments after injection (usually when in excess of 120 milligrams) lasting 2 to 5Â minutes, including tinnitus & audio distortion. As with all injected illicit substances, there is a risk of the user contracting blood-borne infections if sterile injecting equipment is not available or used. Additionally, because cocaine is a vasoconstrictor, and usage often entails multiple injections within several hours or less, subsequent injections are progressively more difficult to administer, which in turn may lead to more injection attempts and more sequelae from improperly performed injection. An injected mixture of cocaine and heroin, known as âspeedball,â is a particularly dangerous combination, as the converse effects of the drugs actually complement each other, but may also mask the symptoms of an overdose. It has been responsible for numerous deaths, including celebrities such as John Belushi, Chris Farley, Mitch Hedberg, River Phoenix and Layne Staley.
Inhalation. Inhalation or smoking involves inhaling cocaine vapor into the lungs by sublimating solid cocaine by heating. Smoking freebase or crack cocaine is most often accomplished using a pipe made from a small glass tube, often taken from "love roses," small glass tubes with a paper rose that are promoted as romantic gifts (Reist 2005). A small piece of clean heavy copper or occasionally stainless steel scouring pad may serve as a reduction base and flow modulator in which the "rock" can be melted and boiled to vapor. Crack often is smoked by placing it at the end of the pipe; a flame held close to it produces vapor, which is then inhaled by the smoker. Powdered cocaine is also sometimes smoked, though heat destroys much of the chemical. Smoking or vaporizing cocaine and inhaling it into the lungs produces an almost immediate "high" that can be very intense quite rapidly. In a Brookhaven National Laboratory medical department study, based on self reports of cocaine abusers who participated in the study, "peak high" was found at mean of 1.4min +/- 0.5Â minutes (Volkow et al. 2000). While the stimulating effects may last for hours, the euphoric sensation is very briefâusually 5 to 15 minutesâprompting the user to smoke more immediately.
Application to skin. Many users rub the powder along the gum line, or onto a cigarette filter, which is then smoked, numbing the gums and teethâhence, the colloquial names of "numbies", "gummers" or "cocoa puffs" for this type of administration. This is mostly done with the small amounts of cocaine remaining on a surface after insufflation. A medical form of cocaine, strictly regulated and available by prescription, is applied to the skin to numb eye, nose, and throat pain (WebMD 2013b).
Oral: Coca leaf chewing and infusions (tea). Unadulterated coca leaves have been chewed for thousands of years in the Andes and remains practiced by millions in South America today (Cortes 2013). Individuals may suck on wads of the leaves and keep them in their cheeks for hours at a time. Coca leaves are typically mixed with an alkaline substance (such as lime) to help dissolve the alkaloids into the saliva and chewed into a wad that is retained in the mouth between gum and cheek (much in the same as chewing tobacco is chewed) and sucked of its juices. The juices are absorbed slowly by the mucous membrane of the inner cheek and by the gastrointestinal tract when swallowed. While the cocaine in the plant has little effect on the unbroken skin, it does act on the mucous membranes of the mouth (as well as the membranes of the eye, nose, and stomach) (Royal Botanic Gardens 1985). However, since the alkaloid cocaine is only in trace amounts in the leaves, it does not cause the euphoric and psychoactive effects associated with use of the concentrated drug. Concentrations vary by variety and region, but leaves have been reported variously as between 0.25% and 0.77% (Plowman and Rivier 1983), between 0.35% and 0.72% by dry weight (Nathanson et al. 1993), and between 0.3% and 1.5% and averaging 0.8% in fresh leaves (Casale and Klein 1993).
Coca leaves also can be boiled to provide a tea. Although coca leaf chewing is common mainly among the indigenous populations, the consumption of coca tea (Mate de coca) is common among all sectors of society in the Andean countries.
Because cocaine is hydrolyzed and rendered inactive in the acidic stomach, it is not readily absorbed when ingested alone. Only when mixed with a highly alkaline substance (such as lime) can it be absorbed into the bloodstream through the stomach. The efficiency of absorption of orally administered cocaine is limited by two additional factors. First, the drug is partly catabolized by the liver. Second, capillaries in the mouth and esophagus constrict after contact with the drug, reducing the surface area over which the drug can be absorbed. Nevertheless, cocaine metabolites can be detected in the urine of subjects that have sipped even one cup of coca leaf infusion. Therefore, this is an actual additional form of administration of cocaine, albeit an inefficient one.
Other methods. An oral method for the psychoactive drug is to wrap up some cocaine in rolling paper and swallow (parachute) it. Little research has been focused on another method: the suppository (anal or vaginal insertion) method of administration, also known as "plugging." This method of administration is commonly administered using an oral syringe. Cocaine can be dissolved in water and withdrawn into an oral syringe which may then be lubricated and inserted into the anus or vagina before the plunger is pushed. The rectum and the vaginal canal is where the majority of the drug would likely be taken up, through the membranes lining its walls.
Uses
Recreational psychoactive drug
Cocaine is best known worldwide for its illegal use as a recreational psychoactive drug. As noted above, this concentrated form of cocaine particularly is used nasally (nasal insufflation is also known as "snorting," "sniffing," or "blowing"), injected, or smoked. In the United States, the development of "crack" cocaine introduced the substance to a generally poorer inner-city market.
The United Nations Office of Drugs and Crime estimated that in 2009, the US cocaine market was $37 billion (and shrinking over the past ten years) and the West and Central European Cocaine market was US$ 33 billion (and increasing over the past ten years) (USODC 2011). According to a 2007 United Nations report, Spain is the country with the highest rate of cocaine usage (3.0% of adults in the previous year) (UNODC 2007). Other countries where the usage rate meets or exceeds 1.5% are the United States (2.8%), England and Wales (2.4%), Canada (2.3%), Italy (2.1%), Bolivia (1.9%), Chile (1.8%), and Scotland (1.5%) (UNODC 2007).
The production, distribution and sale of cocaine products is restricted and/or illegal in most countries. Internationally, it is regulated by the Single Convention on Narcotic Drugs, and the United Nations Convention Against Illicit Traffic in Narcotic Drugs and Psychotropic Substances. In the United States, the manufacture, importation, possession, and distribution of cocaine is additionally regulated by the 1970 Controlled Substances Act. Cocaine is generally treated as a 'hard drug', with severe penalties for possession and trafficking.
Medicine
Strictly regulated, cocaine can be applied externally to the skin to numb pain. Cocaine was historically used as a topical anesthetic in eye and nasal surgery. It is now predominantly used for nasal and lacrimal duct surgery. The major disadvantages of this use are cocaine's intense vasoconstrictor activity and potential for cardiovascular toxicity. Cocaine has since been largely replaced in Western medicine by synthetic local anesthetics such as benzocaine, proparacaine, lignocaine/xylocaine/lidocaine, and tetracaine though it remains available for use if specified. If vasoconstriction is desired for a procedure (as it reduces bleeding), the anesthetic is combined with a vasoconstrictor such as phenylephrine or epinephrine.
In Australia, cocaine is currently prescribed for use as a local anesthetic for conditions such as mouth and lung ulcers. Some ENT specialists occasionally use cocaine within the practice when performing procedures such as nasal cauterization. In this scenario dissolved cocaine is soaked into a ball of cotton wool, which is placed in the nostril for the 10â15Â minutes immediately prior to the procedure, thus performing the dual role of both numbing the area to be cauterized, and vasoconstriction. Even when used this way, some of the used cocaine may be absorbed through oral or nasal mucosa and give systemic effects.
In the United States, cocaine remains an FDA-approved Schedule C-II drug, which can be prescribed by a healthcare provider, but is strictly regulated. A form of cocaine available by prescription is applied to the skin to numb eye, nose, and throat pain and narrow blood vessels (WebMD 2013b).
Unprocessed coca leaf traditionally has been used for a variety of medical purposes, including as a stimulant to overcome fatigue, hunger, and thirst. Because coca constricts blood vessels, it also serves to oppose bleeding, and coca seeds were used for nosebleeds. Coca leaf also has been used to overcome altitude sickness, and in the Andes tourists have been offered coca tea for this purpose (Cortes 2013). In addition, coca extracts have been used as a muscle and cerebral stimulant to alleviate nausea, vomiting, and stomach pains without upsetting digestion (Botany Central 2013; WebMD 2013b). (See the article coca for these and other uses of the coca leaf.)
In the United States, a Stepan Company plant in Maywood, New Jersey manufactures pure cocaine for medical use and also produces a cocaine-free extract of the coca leaf, which is used as a flavoring ingredient in Coca-Cola. Other companies have registrations with the DEA to import coca leaf according to 2011 Federal Register Notices for Importers (ODC 2011), including Johnson Matthey, Inc, Pharmaceutical Materials; Mallinckrodt Inc; Penick Corporation; and the Research Triangle Institute.
History

Coca, the plant in which cocaine is found, has been utilized in unprocessed form for thousands of years. There is archeological evidence that suggests the use of coca leaves 8000 years ago, with the finding of coca leaves of that date (6000 B.C.E.) in floors in Peru, along with pieces of calcite (calcium carbonate), which is used by those chewing leaves to bring out the alkaloids by helping dissolve them into the saliva (Dillehay et al. 2010; Boucher 1991). Coca leaves also have been found in the Huaca Prieta settlement in northern Peru, dated from about 2500 to 1800 B.C.E. (Botany Central 2013; Hurtado 1995). Traces of cocaine also have been in 3000-year-old mummies of the Alto Ramirez culture of Northern Chile, suggesting coca-leaf chewing dates to at least 1500 B.C.E. (Rivera et al. 2005). The remains of coca leaves not only have been found with ancient Peruvian mummies, but pottery from the time period depicts humans with bulged cheeks, indicating the presence of something on which they are chewing (Altman et al. 1985). It is the view of Boucher (1991) that the coca plant was domesticated by 1500 B.C.E. (See coca for more detail on the history of coca.)
The cocaine alkaloid was first isolated by the German chemist Friedrich Gaedcke in 1855. Gaedcke named the alkaloid "erythroxyline" and published a description in the journal Archiv der Pharmazie (Gaedcke 1855).
Cocaine also was isolated in 1859 by Albert Niemann of the University of Göttingen, using an improved purification process. Essentially, three years earlier, in 1856, Friedrich Wöhler asked Dr. Carl Scherzer, a scientist aboard the Novara (an Austrian frigate sent by Emperor Franz Joseph to circle the globe), to bring him a large amount of coca leaves from South America. In 1859, the ship finished its travels and Wöhler received a trunk full of coca. Wöhler passed on the leaves to Albert Niemann, a Ph.D. student at the University of Göttingen in Germany, who then developed the improved process (Niemann 1860).
Niemann described every step he took to isolate cocaine in his dissertation titled Ăber eine neue organische Base in den CocablĂ€ttern (On a New Organic Base in the Coca Leaves), which was published in 1860âit earned him his Ph.D. and is now in the British Library. He wrote of the alkaloid's "colourless transparent prisms" and said that, "Its solutions have an alkaline reaction, a bitter taste, promote the flow of saliva and leave a peculiar numbness, followed by a sense of cold when applied to the tongue."
It was Niemann who named the alkaloid "cocaine," from "coca" (from Quechua "cuca") + suffix "ine" (Niemann 1860). Because of its use as a local anesthetic, a suffix "-caine" was later extracted and used to form names of synthetic local anesthetics.
In 1859, an Italian doctor, Paolo Mantegazza, returned from Peru, where he had witnessed first hand the use of coca by the natives. He proceeded to experiment on himself and upon his return to Milan he wrote a paper in which he described the effects. In this paper he declared coca and cocaine (at the time they were assumed to be the same) as being useful medicinally, in the treatment of "a furred tongue in the morning, flatulence, and whitening of the teeth."

A chemist named Angelo Mariani who read Mantegazza's paper became immediately intrigued with coca and its economic potential. In 1863, Mariani started marketing a wine called Vin Mariani, which had been treated with coca leaves, to become cocawine. The ethanol in wine acted as a solvent and extracted the cocaine from the coca leaves, altering the drink's effect. It contained 6Â mg cocaine per ounce of wine, but Vin Mariani that was to be exported contained 7.2Â mg per ounce, to compete with the higher cocaine content of similar drinks in the United States.
Coca wine (of which Vin Mariani was the best-known brand) and other coca-containing preparations were widely sold as patent medicines and tonics, with claims of a wide variety of health benefits. The original version of Coca-Cola was among these, although the amount in Coca-Cola may have been only trace amounts. Pemberton's original 1886 recipe for Coca-Cola noted a "pinch of coca leaves." By 1891, just five years later, the amount of cocaine was significantly cut, although the ingredient was left in in order to protect the trade name of Coca-Cola. By 1902, it was held that Coca-Cola contained a little as 1/400th of a grain of cocaine per ounce of syrup. In 1929, Coca-Cola became cocaine-free, but before then it was estimated that the amount of cocaine already was no more than one part in 50 million (Mikkelson 2011; Liebowitz 1983; Cortes 2013).
In 1879, cocaine began to be used to treat morphine addiction.
Also in 1879, Vassili von Anrep, of the University of WĂŒrzburg devised an experiment to demonstrate the analgesic properties of the newly discovered alkaloid. He prepared two separate jars, one containing a cocaine-salt solution and the other containing merely salt water. He then submerged a frog's legs into the two jars, one leg in the treatment and one in the control solution, and proceeded to stimulate the legs in several different ways. The leg that had been immersed in the cocaine solution reacted very differently from the leg that had been immersed in salt water (Yentis and Vlassakov 1999).
Karl Koller experimented with cocaine for ophthalmic usage. In an infamous experiment in 1884, he experimented upon himself by applying a cocaine solution to his own eye and then pricking it with pins. His findings were presented to the Heidelberg Ophthalmological Society. Also in 1884, Jellinek demonstrated the effects of cocaine as a respiratory system anesthetic.
Cocaine was introduced into clinical use as a local anesthetic in Germany in 1884, about the same time as Sigmund Freud published his work Ăber Coca, in which he wrote that cocaine causes:
Exhilaration and lasting euphoria, which in no way differs from the normal euphoria of the healthy person. You perceive an increase of self-control and possess more vitality and capacity for work. In other words, you are simply normal, and it is soon hard to believe you are under the influence of any drug. Long intensive physical work is performed without any fatigue. This result is enjoyed without any of the unpleasant after-effects that follow exhilaration brought about by alcohol. Absolutely no craving for the further use of cocaine appears after the first, or even after repeated taking of the drug.
In 1885, William Halsted demonstrated nerve-block anesthesia (Halsted 1885), and James Leonard Corning demonstrated peridural anesthesia (Corning 1885).
In 1885, the U.S. manufacturer Parke-Davis sold cocaine in various forms, including cigarettes, powder, and even a cocaine mixture that could be injected directly into the user's veins with the included needle. The company promised that its cocaine products would "supply the place of food, make the coward brave, the silent eloquent and render the sufferer insensitive to pain."
In 1898, Heinrich Quincke demonstrated the use of cocaine for spinal anesthesia.
The first synthesis and elucidation of the structure of the cocaine molecule was by Richard WillstÀtter in 1898 (Humphrey and O'Hagan 2001). The synthesis started from tropinone, a related natural product and took five steps.
In the early 20th century, products with cocaine became illegal in most countries outside of South America, after the addictive nature of cocaine was widely recognized.
In the United States, the federal government instituted a national labeling requirement for cocaine and cocaine-containing products through the Food and Drug Act of 1906. The next impactful federal regulation was the Harrison Narcotics Tax Act of 1914. While this act is often seen as the start of prohibition, the act itself was not actually a prohibition on cocaine, but instead setup a regulatory and licensing regime. The Harrison Act left manufacturers of cocaine untouched so long as they met certain purity and labeling standards.Despite that cocaine was typically illegal to sell and legal outlets were more rare, the quantities of legal cocaine produced declined very little. Legal cocaine quantities did not decrease until the Jones-Miller Act of 1922 put serious restrictions on cocaine manufactures (Madge 2001; Gootenberg 1999).
As of 2012, Peru was the leading producer of pure cocaine, followed by Bolivia and Colombia. Colombia had been the leading producer for over a decade, producing three-quarters of the world's annual yield, but the U.S. launched at $7.5 billion effort in 1999 toward helping the Colombia government crack down on drug organizations and insurgencies. Peru had been the leading producer in the 1980s and 1990s (NBC 2012).
Notes
- â 1.0 1.1 K. Fattinger, N. L. Benowitz, R. T. Jones, and D. Verotta, "Nasal Mucosal Versus Gastrointestinal Absorption of Nasally Administered Cocaine," Eur. J. Clin. Pharmacol. 56, iss. 4(2000): 305â10. PMID 10954344.
- â G. Barnett, R. Hawks, and R. Resnick, "Cocaine Pharmacokinetics in Humans," J Ethnopharmacol 3, issue 2â3(1981): 353â66. PMID 7242115.
- â A. R. Jeffcoat, M. Perez-Reyes, J. M. Hill, B. M. Sadler, and C. E. Cook, "Cocaine Disposition in Humans After Intravenous Injection, Nasal Insufflation (Snorting), or Smoking," Drug Metab. Dispos. 17, iss. 2(1989): 153â9. PMID 2565204.
- â P. Wilkinson, C. Van Dyke, P. Jatlow, P. Barash, and R. Byck, "Intranasal and Oral Cocaine Kinetics," Clin. Pharmacol. Ther. 27, iss. 3(1980): 386â94. PMID 7357795.
- â D. Nutt, L. A. King, W. Saulsbury, and C. Blakemore, "Development of a Rational Scale to Assess the Harm of Drugs of Potential Misuse". The Lancet 369, iss. 9566 (2007): 1047â1053. Retrieved September 3, 2013
- â Vin Mariani Winery, "Experience Vin Mariani Today," Cocanaturally.com. Retrieved September 11, 2013.
ReferencesISBN links support NWE through referral fees
- Aggrawal, A. 1995. Narcotic Drugs. India: National Book Trust. ISBN 9788123713830.
- Altman, A. J., D. M. Albert, and G. A. Fournier. 1985. Cocaine's use in ophthalmology: Our 100-year heritage. Surv Ophthalmol 29(4): 300â6. PMID 3885453. Retrieved September 11, 2013.
- Baigent, M. 2003. Physical complications of substance abuse: What the psychiatrist needs to know. Curr Opin Psychiatry 16(3): 291â296.
- Barnett, G., R. Hawks, and R. Resnick. 1981. Cocaine pharmacokinetics in humans. Journal of Ethnopharmacology 3(2â3): 353â66. PMID 7242115. Retrieved September 17, 2013.
- Bonkovsky, H. L., and S. Mehta. 2001. Hepatitis C: A review and update. J. Am. Acad. Dermatol. 44(2): 159â82. PMID 11174373. Retrieved September 17, 2013.
- Botany Central. 2013. Erythroxylum coca: The coca plant. Botany Central. Retrieved August 6, 2013.
- Boucher, D. H. 1991. Cocaine and the coca plant. BioScience 41(2): 72-76.
- Carta, M., A. M. Allan, L. D. Partridge, and C. F. Valenzuela. 2003. Cocaine inhibits 5-HT3 receptor function in neurons from transgenic mice overexpressing the receptor. Eur. J. Pharmacol. 459(2â3): 167â9. PMID 12524142.
- Casale, J. F., and R. F. X. Klein. 1993. Illicit production of cocaine. Forensic Science Review 5: 95-107. Retrieved August 14, 2013.
- Corning, J. L. 1885. An experimental study. New York Medical Journal 42: 483.
- Cortes, R. 2013. The condemned coca leaf. NY Daily News January 13, 2013. Retrieved August 11, 2013.
- D'haenen, H. A. H., J. A. den Boer, and P. Willner. 2002. Biological Psychiatry. Wiley. ISBN 9780471491989. Retrieved September 17, 2013.
- Dietrich, J. B. 2009. Alteration of blood-brain barrier function by methamphetamine and cocaine. Cell and tissue research 336(3): 385â392. PMID 19350275.
- Dillehay, T. D., J. Rossen, D. Ugent, A. Karathanasis, V. VĂĄsquez, and P. J. Netherly. 2010. Early Holocene coca chewing in northern Peru. Antiquity 84(326): 939â953. Retrieved August 14, 2013.
- Fandiño, A. S., S. W. Toennes, and G. F. Kauert. 2002. Studies on hydrolytic and oxidative metabolic pathways of anhydroecgonine methyl ester (methylecgonidine) using microsomal preparations from rat organs. Chem. Res. Toxicol. 15(12): 1543â8. PMID 12482236.
- Filip, M., M. J. Bubar, and K. A. Cunningham. 2004. Contribution of serotonin (5-hydroxytryptamine; 5-HT) 5-HT2 receptor subtypes to the hyperlocomotor effects of cocaine: acute and chronic pharmacological analyses. J. Pharmacol. Exp. Ther. 310(3): 1246â54. PMID 15131246.
- Gaedcke, F. 1855. Ueber das Erythroxylin, dargestellt aus den BlĂ€ttern des in SĂŒdamerika cultivirten Strauches Erythroxylon coca Lam. Archiv der Pharmazie 132(2): 141-150. Retrieved September 11, 2013.
- Gootenberg, P. (Ed.) 1999. Cocaine: Global Histories. London: Routledge. ISBN 0203026462.
- Halsted, W. 1885. Practical comments on the use and abuse of cocaine. New York Medical Journal 42: 294â295.
- Humphrey, A. J., and D. O'Hagan. 2001. Tropane alkaloid biosynthesis. A century old problem unresolved. Nat Prod Rep 18(5): 494â502. PMID 11699882. Retrieved September 11, 2013.
- Hurtado, J. 1995. Cocaine the Legend: About Coca and Cocaine La Paz, Bolivia: Accion Andina, ICORI.
- Inciardi,J. A. 1992. The War on Drugs II: The Continuing Epic of Heroin, Cocaine, Crack, Crime, AIDS, and Public Policy. Mayfield. ISBN 1559340169.
- Jaffe, J. A., and P. L. Kimmel. 2006. Chronic nephropathies of cocaine and heroin abuse: A critical review. Clinical Journal of the American Society of Nephrology 1(4): 655-667. PMID 17699270. Retrieved September 17, 2013.
- Jeffrey, S., and C. Vega. 2007. Stimulant abuse may increase stroke among young adults. Medscape. Retrieved September 17, 2013.
- Kolbrich, E. A., A. J. Barnes, D. A. Gorelick, S. J. Boyd, E. J. Cone, and M. A. Huestis. 2006. Major and minor metabolites of cocaine in human plasma following controlled subcutaneous cocaine administration. J Anal Toxicol 30(8): 501â10. PMID 17132243. Retrieved September 17, 2013.
- Liebowitz, M. R. 1983. The Chemistry of Love. Boston: Little, Brown, & Co. ISNB 0316524301.
- Lluch, J., M. RodrĂguez-Arias, M. A. Aguilar,and J. Miñarro. 2005. Role of dopamine and glutamate receptors in cocaine-induced social effects in isolated and grouped male OF1 mice. Pharmacol. Biochem. Behav. 82(3): 478â87. PMID 16313950. Retrieved September 17, 2013.
- Lowinson, J. H., P. Ruiz, and R. B. Millman. 2004. Substance abuse: A comprehensive textbook, 4th edition. Lippincott Williams & Wilkins. ISBN 9780781734745. Retrieved September 17, 2013.
- Madge, T. 2001. White Mischief: A Cultural History of Cocaine. Edinburgh: Mainstream Publishing Company. ISBN 1840184051.
- Mikkelson, B. 2011. Cocaine-Cola. Snopes.com. Retrieved August 13, 2013.
- Moore, P. M.,and B. Richardson. 1998. Neurology of the vasculitides and connective tissue diseases. J. Neurol. Neurosurg. Psychiatr. 65(1): 10â22. PMID 9667555. Retrieved September 17, 2013.
- Nainggolan, L. 2010. Cocaine-related sudden death not so rare. Heartwire. Retrieved Sept. 17, 2013
- Nathanson, J. A., E. J. Hunnicutt, L. Kantham, and C. Scavone. 1993. Cocaine as a naturally occurring insecticide. Proc. Nat. Acad. Sci. 90: 9645-9648. Retrieved September 17, 2013.
- National Institutes of Health/National Institute On Drug Abuse (NIH/NIDA). 2003. Sigma receptors play role in cocaine-induced suppression of immune system. ScienceDaily. Retrieved September 5, 2013.
- NBC Worldnews. 2012. Peru overtakes Colombia as top cocaine producer. NBC News July 31, 2012. Retrieved September 17, 2013.
- Nelson, G. 1998. Hip Hop America. New York: Viking Penguin. ISBN 0670871532.
- Niemann, A. 1860. Ueber eine neue organische Base in den CocablÀttern. Archiv der Pharmazie 153(2): 129-256. Retrieved September 11, 2013.
- Nutt, D., L. A. King, W. Saulsbury, and C. Blakemore, Development of a rational scale to assess the harm of drugs of potential misuse. The Lancet 369, iss. 9566 (2007): 1047â1053. Retrieved September 3, 2013
- Office of Diversion Control (ODC). 2011. Importers Notice of Registration - 2011. Drug Enforcement Agency, U.S. Department of Justice. Retrieved August 14, 2013.
- Pagliaro, L., and A. M. Pagliaro. 2004. Pagliarosâ Comprehensive Guide to Drugs and Substances of Abuse. Washington, D.C.: American Pharmacists Association. ISBN 9781582120669.
- Peces, R., R. A. Navascués, J. Baltar, M. Seco, and J. Alvarez. 1999. Antiglomerular basement membrane antibody-mediated glomerulonephritis after intranasal cocaine use. Nephron 81(4): 434-438. PMID 10095180. Retrieved September 17, 2013.
- Plowman, T, and L. Rivier. 1983. Cocaine and Cinnamoylcocaine content of thirty-one species of Erythroxylum (Erythroxylaceae)". Annals of Botany 51: 641â659.
- Reist, M. 2005. A rose by another name: Crack pipe. Lincoln Journal Star January 16, 2005. Retrieved September 17, 2013.
- Ries, R. K., S. C. Miller, and D. A. Fiellin. 2009. Principles of Addiction Medicine. Lippincott Williams & Wilkins. ISBN 0781774772.
- Rivera, M. A., A. C. Aufderheide, L. W. Cartmell, C. M. Torres, and O. Langsjoen. 2005. Antiquity of coca-leaf chewing in the south central Andes: A 3,000 year archaeological record of coca-leaf chewing from northern Chile. Journal of Psychoactive Drugs 37(4): 455â458. Retrieved August 14, 2013.
- Royal Botanic Gardens, Kew. 1985. Entry for Erythroxylum coca Lam. [family ERYTHROXYLACEAE]. JSTOR. Retrieved August 6, 2013.
- Scheidweiler, K. B., M. A. Plessinger, J. Shojaie, R. W. Wood, and T. C. Kwong. 2003. Pharmacokinetics and pharmacodynamics of methylecgonidine, a crack cocaine pyrolyzate. Journal of Pharmacology and Experimental Therapeutics 307(3): 1179-87. PMID 14561847. Retrieved September 17, 2013.
- Sharma, H. S., D. Muresanu, A. Sharma, and R. Patnaik. 2009. Cocaine-induced breakdown of the blood brain barrier and neurotoxicity. International Review of Neurobiology 88: 297â334. PMID 19897082. Retrieved September 17, 2013.
- Spanagel, R., and F. Weiss. 1999. The dopamine hypothesis of reward: Past and current status. Trends Neurosci. 22(11): 521â7. PMID 10529820. Retrieved September 17, 2013.
- Spillane, J. F. 2000. Cocaine: From Medical Marvel to Modern Menace in the United States, 1884â1920. Baltimore and London: The John Hopkins University Press. ISBN 0801862302.
- Trozak, D.,and W. Gould. 1984. Cocaine abuse and connective tissue disease. J Am Acad Dermatol 10(3): 525. PMID 6725666. Retrieved September 17, 2013.
- United Nations Office of Drugs and Crime (USODC). 2007. 2007 World Drug Report. United Nations. Retrieved September 17, 2013.
- United Nations Office of Drugs and Crime (USODC). 2011. The transatlantic cocaine market: Research paper. United Nations. Retrieved August 13, 2013.
- van der Woude, F. J. 2000. Cocaine use and kidney damage. Nephrology Dialysis Transplantation 15(3): 299â301. PMID 10692510. Retrieved September 17, 2013.
- Vasica, G., and C. C. Tennant. 2002. Cocaine use and cardiovascular complications. Med. J. Aust. 177(5): 260â2. PMID 12197823.
- Volkow, N. D., G.-J. Wang, M. W. Fischman, R. Foltin, J. S. Fowler, D. Franceschi, M. Franceschi, J. Logan, and S. J. Gatley. 2000. Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sciences 67(12): 1507-1515. PMID 10983846.
- WebMD. 2013a. Cocaine use and its effects. WebMD. Retrieved August 12, 2013.
- WebMD. 2013b. Find a vitamin or supplement: Coca. WebMD. Retrieved August 13, 2013.
- World Health Organization (WHO). 2004. Neuroscience of Psychoactive Substance Use and Dependence. World Health Organization. Retrieved September 17, 2013.
- World Health Organization (WHO). 2007. International Medical Guide for Ships. World Health Organization. Retrieved September 17, 2013.
- Yang, Y., Q. Ke, J. Cai, Y. F. Xiao, and J. P. Morgan. 2001. Evidence for cocaine and methylecgonidine stimulation of M2 muscarinic receptors in cultured human embryonic lung cells. Br. J. Pharmacol. 132(2): 451â60. PMID 11159694. Retrieved September 17, 2013.
- Yentis, S. M.,and K. V. Vlassakov. 1999. Vassily von Anrep, forgotten pioneer of regional anesthesia. Anesthesiology 90(3): 890â5. PMID 10078692.
- Zhao, W. 2008. Mechanisms Mediating Sex Differences in the Effects of Cocaine. ProQuest. ISBN 0549994580. Retrieved September 17, 2013.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.