Difference between revisions of "Glycine" - New World Encyclopedia
Rick Swarts (talk | contribs) |
Rick Swarts (talk | contribs) |
||
| Line 11: | Line 11: | ||
{{for|the plant|Glycine (plant)}} | {{for|the plant|Glycine (plant)}} | ||
| − | '''Glycine''' is the simplest of the α-[[amino acid | + | '''Glycine''' is the simplest of the α-[[amino acid]]s in terms of molecular structure. It is one of the 20 most common, natural, "proteinogenic" (literally, protein building) amino acids ([[amino acid#Standard amino acids|standard amino acids]]. Glycine is unique among these in that is not [[optical isomerism|optically active]]; that is, it does not have occur in two possible optical isomers, called D and L. |
While glycine is necessary for normal functioning in humans, it is categorized as a [[amino acid#essential amino acid|non-essential amino acid]] since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. | While glycine is necessary for normal functioning in humans, it is categorized as a [[amino acid#essential amino acid|non-essential amino acid]] since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. | ||
| Line 37: | Line 37: | ||
==Biosynthesis== | ==Biosynthesis== | ||
| − | Glycine is not essential to the human diet, since it is synthesized in the body. It is biosynthesized from the amino acid [[serine]]. The enzyme serine hydroxymethyl transferase catalyses this transformation | + | Glycine is not essential to the human diet, since it is synthesized in the body. It is biosynthesized from the amino acid [[serine]]. The enzyme serine hydroxymethyl transferase catalyses this transformation (Lehninger et al. 2000): |
| + | |||
: HO<sub>2</sub>CCH(NH<sub>2</sub>)CH<sub>2</sub>OH + [[Folic acid|H<sub>2</sub>folate]] → HO<sub>2</sub>CCH<sub>2</sub>NH<sub>2</sub> + CH<sub>2</sub>-folate + H<sub>2</sub>O | : HO<sub>2</sub>CCH(NH<sub>2</sub>)CH<sub>2</sub>OH + [[Folic acid|H<sub>2</sub>folate]] → HO<sub>2</sub>CCH<sub>2</sub>NH<sub>2</sub> + CH<sub>2</sub>-folate + H<sub>2</sub>O | ||
==Physiological function== | ==Physiological function== | ||
| − | |||
| − | |||
| − | + | In addition to the building block of [[protein]]s, glycine is a building block to numerous other chemical compounds. For one, glycine is one of the reactants in the synthesis of porphyrins such as heme, which is a component of the [[hemoglobin]] molecules found in [[red blood cell]]s. Specifically, [[aminolevulinic acid]], the key precursor to [[porphyrin]]s, is biosynthesized from glycine and succinoyl [[coenzyme A]]. Glycine provides the central C<sub>2</sub>N subunit of all [[purine]]s (Lehninger et al. 2000). | |
| − | |||
| − | + | Glycine also is important in the biosynthesis of the amino acid serine and the [[enzyme|coenzyme]] glutathione. | |
| − | |||
| − | + | Glycine is one of the major inhibitory [[neurotransmitter]]s in the [[central nervous system]], especially in the [[spinal cord]], brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potential (IPSP). [[Strychnine]] is an antagonist at ionotropic glycine receptors. Glycine is a required co-agonist, along with [[glutamate]], for [[NMDA receptor]]s. In contrast to the inhibitory role of glycine in the spinal cord, this behavior is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The [[LD50]] of glycine is 7930 mg/kg in rats (oral) (PTCL 2006), and it usually causes death by [[hyperexcitability]]. | |
| − | + | Glycine is also important as a additive for animal feed, used to reduce saccharin's bitter taste, and used in biochemical research and medicine. | |
==Silk== | ==Silk== | ||
| − | + | Glycine is a key component in [[silk]]. Spider silk is a remarkably strong material, with a tensile strength is comparable to that of high-grade [[steel]] (Shao and Vollrath 2002). It has been said that a circular web, similar in all ways to that found in nature but the size of a football field, could stop a commercial jetliner in mid flight (Henke 2007), and yet it is so light that a single strand strand long enough to circle the earth would weigh less than 16 ounces (460 g). | |
| − | |||
| − | + | Spider dragline silk is made up of the protein fibroin, which is a combination of the proteins spidroin 1 and spidroin 2. The bulk of these proteins are made up of glycine (Gly) and [[alanine]] (Ala), with the remaining components mostly the amino acids [[proline]] (Pro), [[tyrosine]] (Tyr), [[arginine]] (Arg), [[glutamine]] (Gln), [[serine]] (Ser), and [[leucine]] (Leu) (UB 2007). Spidroin 1 and 2 are made up of polyalanine regions with about 4 to 9 alanine monomers in a block (van Beek et al. report approximately 8 monomers) and glycine rich areas with a sequence of five amino acids continuously repeated, such as Gly-Pro-Gly-Gln-Gln (van Beek et al. 2002; UB 2007). | |
| − | |||
| − | Spider dragline silk is made up of the protein fibroin, which is a combination of the proteins spidroin 1 and spidroin 2. The bulk of these proteins are made up of | ||
The general trend in spider silk structure thus is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form crystals, whereas the other segments form [[amorphous]] domains. It is the interplay between the hard crystalline segments, and the elastic semi- amorphous regions, that gives spider silk its extraordinary properties. The fact that the major amino acids in spider silk are the two smallest amino acids, and lack bulky side groups, allows them to pack together tightly (UB 2007). | The general trend in spider silk structure thus is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form crystals, whereas the other segments form [[amorphous]] domains. It is the interplay between the hard crystalline segments, and the elastic semi- amorphous regions, that gives spider silk its extraordinary properties. The fact that the major amino acids in spider silk are the two smallest amino acids, and lack bulky side groups, allows them to pack together tightly (UB 2007). | ||
| Line 67: | Line 61: | ||
==References== | ==References== | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | + | * Dawson, R. M. C., D. C. Elliott, W. H. Elliott, and K. M. Jones. 1986. ''Data for Biochemical Research'', 3rd edition. Oxford : Clarendon Press. ISBN 0198553587. | |
| + | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319. | ||
* Henke, B. 2007. [http://www.poststar.com/articles/2007/06/10/sports/columns/outdoors-bhenke/74c4533dc34bd13d852572f6000217fc.txt The webs they weave]. ''Poststar.com'' Sunday, June 10, 2007. Retrieved June 15, 2007. | * Henke, B. 2007. [http://www.poststar.com/articles/2007/06/10/sports/columns/outdoors-bhenke/74c4533dc34bd13d852572f6000217fc.txt The webs they weave]. ''Poststar.com'' Sunday, June 10, 2007. Retrieved June 15, 2007. | ||
* International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | ||
* Kendall, E. C., and B. F. McKenzie. 1941. [http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf dl-Alanine]. ''Organic Syntheses'' 1: 21. | * Kendall, E. C., and B. F. McKenzie. 1941. [http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf dl-Alanine]. ''Organic Syntheses'' 1: 21. | ||
| + | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry'', 3rd ed. New York: Worth Publishing. ISBN 1572591536. | ||
| + | * Physical & Theoretical Chemistry Laboratory (PTCL). 2006. [http://physchem.ox.ac.uk/MSDS/GL/glycine.html Safety (MSDS) data for glycine]. ''The Physical and Theoretical Chemistry Laboratory, Oxford University''. Retrieved June 14, 2007. | ||
* Shao, Z., and F. Vollrath. 2002. Surprising strength of silkworm silk. ''Nature'' 418: 741. | * Shao, Z., and F. Vollrath. 2002. Surprising strength of silkworm silk. ''Nature'' 418: 741. | ||
* University of Bristol, School of Chemistry (UB). 2007. [http://www.chm.bris.ac.uk/motm/spider/page3.htm Spider silk: Chemical structure]. ''University of Bristol''. Retrieved June 14, 2007. | * University of Bristol, School of Chemistry (UB). 2007. [http://www.chm.bris.ac.uk/motm/spider/page3.htm Spider silk: Chemical structure]. ''University of Bristol''. Retrieved June 14, 2007. | ||
| Line 83: | Line 75: | ||
==External links== | ==External links== | ||
| − | *[http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrugs/gly_0127.shtml | + | *[http://www.pdrhealth.com/drug_info/nmdrugprofiles/nutsupdrugs/gly_0127.shtml Glycine]. Retrieved June 15, 2007. |
| − | *[http://www.compchemwiki.org/index.php?title=Glycine Computational Chemistry Wiki] | + | *[http://www.compchemwiki.org/index.php?title=Glycine Computational Chemistry Wiki]. Retrieved June 15, 2007. |
| − | *[http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/GlyCleave.html Glycine cleavage system] | + | *[http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/GlyCleave.html Glycine cleavage system]. Retrieved June 15, 2007. |
| − | + | ||
{{AminoAcids}} | {{AminoAcids}} | ||
Revision as of 22:49, 15 June 2007
| |
Glycine | |
| Systematic (IUPAC) name | |
| aminoethanoic acid | |
| Identifiers | |
| CAS number | 56-40-6 |
| PubChem | 750 |
| Chemical data | |
| Formula | C2H5NO2 |
| Mol. weight | 75.07 |
| SMILES | NCC(O)=O |
| Complete data | |
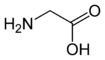
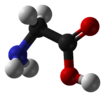
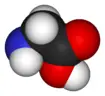
Glycine is the simplest of the α-amino acids in terms of molecular structure. It is one of the 20 most common, natural, "proteinogenic" (literally, protein building) amino acids (standard amino acids. Glycine is unique among these in that is not optically active; that is, it does not have occur in two possible optical isomers, called D and L.
While glycine is necessary for normal functioning in humans, it is categorized as a non-essential amino acid since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions.
Most proteins contain only small quantities of glycine. A notable exception is collagen, which contains about one-third glycine. Glycine is a major component of silk, along with alanine, as well as gelatin.
Glycine's three letter code is GLY, its one letter code is G, and its codons are GGU, GGC, GGA and GGG (IUPAC-IUB 1983). Its systematic name is aminoethanoic acid. systematic name
Structure
Note:In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. Glycine, however, because of its simple structure and two hydrogen atoms at the α carbon, does not have D- and L-stereoisomers. It is unqiue among the 20 standard amino acids in not being optically active.
Glycine is the smallest α-amino acid, is structurally simple (having a side chain of just hydrogen), rotates easily, and adds flexibility to the protein chain. It is able to fit into the tightest spaces, e.g., the triple helix of collagen. Because of its structural simplicity, this compact amino acid tends to be evolutionarily conserved in, for example, cytochrome c, myoglobin, and hemoglobin.
Glycine has the chemical formula CH2(NH2)-COOH, or more generally, C2H5NO2.
Biosynthesis
Glycine is not essential to the human diet, since it is synthesized in the body. It is biosynthesized from the amino acid serine. The enzyme serine hydroxymethyl transferase catalyses this transformation (Lehninger et al. 2000):
- HO2CCH(NH2)CH2OH + H2folate → HO2CCH2NH2 + CH2-folate + H2O
Physiological function
In addition to the building block of proteins, glycine is a building block to numerous other chemical compounds. For one, glycine is one of the reactants in the synthesis of porphyrins such as heme, which is a component of the hemoglobin molecules found in red blood cells. Specifically, aminolevulinic acid, the key precursor to porphyrins, is biosynthesized from glycine and succinoyl coenzyme A. Glycine provides the central C2N subunit of all purines (Lehninger et al. 2000).
Glycine also is important in the biosynthesis of the amino acid serine and the coenzyme glutathione.
Glycine is one of the major inhibitory neurotransmitters in the central nervous system, especially in the spinal cord, brainstem, and retina. When glycine receptors are activated, chloride enters the neuron via ionotropic receptors, causing an Inhibitory postsynaptic potential (IPSP). Strychnine is an antagonist at ionotropic glycine receptors. Glycine is a required co-agonist, along with glutamate, for NMDA receptors. In contrast to the inhibitory role of glycine in the spinal cord, this behavior is facilitated at the (NMDA) glutaminergic receptors which are excitatory. The LD50 of glycine is 7930 mg/kg in rats (oral) (PTCL 2006), and it usually causes death by hyperexcitability.
Glycine is also important as a additive for animal feed, used to reduce saccharin's bitter taste, and used in biochemical research and medicine.
Silk
Glycine is a key component in silk. Spider silk is a remarkably strong material, with a tensile strength is comparable to that of high-grade steel (Shao and Vollrath 2002). It has been said that a circular web, similar in all ways to that found in nature but the size of a football field, could stop a commercial jetliner in mid flight (Henke 2007), and yet it is so light that a single strand strand long enough to circle the earth would weigh less than 16 ounces (460 g).
Spider dragline silk is made up of the protein fibroin, which is a combination of the proteins spidroin 1 and spidroin 2. The bulk of these proteins are made up of glycine (Gly) and alanine (Ala), with the remaining components mostly the amino acids proline (Pro), tyrosine (Tyr), arginine (Arg), glutamine (Gln), serine (Ser), and leucine (Leu) (UB 2007). Spidroin 1 and 2 are made up of polyalanine regions with about 4 to 9 alanine monomers in a block (van Beek et al. report approximately 8 monomers) and glycine rich areas with a sequence of five amino acids continuously repeated, such as Gly-Pro-Gly-Gln-Gln (van Beek et al. 2002; UB 2007).
The general trend in spider silk structure thus is a sequence of amino acids (usually alternating glycine and alanine, or alanine alone) that self-assemble into a beta sheet conformation. These "Ala rich" blocks are separated by segments of amino acids with bulky side-groups. The beta sheets stack to form crystals, whereas the other segments form amorphous domains. It is the interplay between the hard crystalline segments, and the elastic semi- amorphous regions, that gives spider silk its extraordinary properties. The fact that the major amino acids in spider silk are the two smallest amino acids, and lack bulky side groups, allows them to pack together tightly (UB 2007).
The glycine-rich regions give spider silk its elasticity, as each sequence of five amino acids is followed by a 180 degree turn, resulting in a spiral. Capture silk is the most elastic, with about 43 repeats on average, and can extend 2 to 4 times its original length, while dragline silk only repeats about 9 times and can extend about 30% of original length (UB 2007).
ReferencesISBN links support NWE through referral fees
- Dawson, R. M. C., D. C. Elliott, W. H. Elliott, and K. M. Jones. 1986. Data for Biochemical Research, 3rd edition. Oxford : Clarendon Press. ISBN 0198553587.
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- Henke, B. 2007. The webs they weave. Poststar.com Sunday, June 10, 2007. Retrieved June 15, 2007.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Kendall, E. C., and B. F. McKenzie. 1941. dl-Alanine. Organic Syntheses 1: 21.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
- Physical & Theoretical Chemistry Laboratory (PTCL). 2006. Safety (MSDS) data for glycine. The Physical and Theoretical Chemistry Laboratory, Oxford University. Retrieved June 14, 2007.
- Shao, Z., and F. Vollrath. 2002. Surprising strength of silkworm silk. Nature 418: 741.
- University of Bristol, School of Chemistry (UB). 2007. Spider silk: Chemical structure. University of Bristol. Retrieved June 14, 2007.
- van Beek, J. D., S. Hess, F. Vollrath, and B. H. Meier. 2002. The molecular structure of spider dragline silk: Folding and orientation of the protein backbone. Proc Natl Acad Sci USA 99(16): 10266-10271. Retrieved June 14, 2007.
External links
- Glycine. Retrieved June 15, 2007.
- Computational Chemistry Wiki. Retrieved June 15, 2007.
- Glycine cleavage system. Retrieved June 15, 2007.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.