Difference between revisions of "Glucose" - New World Encyclopedia
| Line 41: | Line 41: | ||
|- | |- | ||
|} | |} | ||
| − | '''Glucose''' (Glc) is a [[monosaccharide]] (or ''simple sugar'') with the chemical formula [[Carbon|C]]<sub>6</sub>[[Hydrogen|H]]<sub>12</sub>[[Oxygen|O]]<sub>6</sub>. It is the major free sugar circulating in the blood of higher animals, and the preferred fuel of the brain and nervous system, as well as red blood cells. | + | '''Glucose''' (Glc) is a [[monosaccharide]] (or ''simple sugar'') with the chemical formula [[Carbon|C]]<sub>6</sub>[[Hydrogen|H]]<sub>12</sub>[[Oxygen|O]]<sub>6</sub>. It is the major free sugar circulating in the blood of higher animals, and the preferred fuel of the [[brain]] and [[nervous system]], as well as red blood cells ([[erythrocytes]]). |
| − | + | As a universal [[substrate]] for the production of cellular energy, glucose is of central importance in the [[metabolism]] of all life forms. It is one of the main products of [[photosynthesis]], the process by which [[photoautotrophs]] such as [[plants]] and [[algae]] convert energy from sunlight into chemical energy to be used by the cell. Glucose is also a major starting point for [[cellular respiration]], in which the chemical bonds of energy-rich molecules such as glucose are converted into energy usable for life processes. | |
All major dietary [[carbohydrates]] contain glucose, either as their only building block, as in [[starch]] and [[glycogen]], or in combination with another monosaccharide, as in [[sucrose]] (“table sugar”) and [[lactose]], the primary sugar found in milk. | All major dietary [[carbohydrates]] contain glucose, either as their only building block, as in [[starch]] and [[glycogen]], or in combination with another monosaccharide, as in [[sucrose]] (“table sugar”) and [[lactose]], the primary sugar found in milk. | ||
| Line 52: | Line 52: | ||
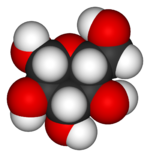
[[Image:Beta-D-glucose-3D-vdW.png|thumb|left|150px|A space-filling model of glucose.]] | [[Image:Beta-D-glucose-3D-vdW.png|thumb|left|150px|A space-filling model of glucose.]] | ||
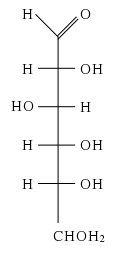
[[Image:D-glucose.svg|frame|right|The open-chain form of <small>D</small>-glucose.]] | [[Image:D-glucose.svg|frame|right|The open-chain form of <small>D</small>-glucose.]] | ||
| − | Sugars are classified according to two properties: (1) | + | Sugars are classified according to two properties: (1) number of carbon atoms and (2) type of [[functional group]] (either an aldehyde or a ketone group). Glucose, which has six [[carbon]] [[atom]]s (i.e., it is a [[hexose]] sugar) and contains an [[aldehyde]] group (-CHO), is thus referred to as an ''aldohexose''. |
The glucose molecule can exist in an open-chain (acyclic) form and a ring (cyclic) form. In solution and at neutral pH, the cyclic form is predominant at [[equilibrium]]. When glucose exists in cyclic form, the functional group is not free, making the molecule less reactive. This preference for the less reactive ring form offers a possible explanation for the crucial and widespread use of glucose in metabolism, as opposed to another monosaccharide such as [[fructose]] (Fru). The low tendency of glucose, in comparison to other hexose sugars, to non-specifically react with the [[amino]] groups of [[proteins]] might explain its importance to advanced life. | The glucose molecule can exist in an open-chain (acyclic) form and a ring (cyclic) form. In solution and at neutral pH, the cyclic form is predominant at [[equilibrium]]. When glucose exists in cyclic form, the functional group is not free, making the molecule less reactive. This preference for the less reactive ring form offers a possible explanation for the crucial and widespread use of glucose in metabolism, as opposed to another monosaccharide such as [[fructose]] (Fru). The low tendency of glucose, in comparison to other hexose sugars, to non-specifically react with the [[amino]] groups of [[proteins]] might explain its importance to advanced life. | ||
| Line 60: | Line 60: | ||
==Natural sources of glucose== | ==Natural sources of glucose== | ||
| − | *''The conversion of light energy into chemical energy''. Glucose is one of the products of photosynthesis in plants and some [[ | + | *''The conversion of light energy into chemical energy''. Glucose is one of the products of photosynthesis in plants and algae, as well as some [[bacteria]] and [[protists]]. |
| − | *''The breakdown of storage forms of glucose''. Glucose can be obtained through the breakdown of glycogen, the storage form of glucose in animals and [[fungi]], through a process known as [[glycogenolysis]]. Glycogen is | + | *''The breakdown of storage forms of glucose''. Glucose can be obtained through the breakdown of glycogen, the storage form of glucose in animals and [[fungi]], through a process known as [[glycogenolysis]]. Glycogen is an auxiliary energy source, tapped and converted back into glucose when there is need for energy. In plants, glucose is stored as [[starch]]. |
| − | *''The synthesis of glucose from non-carbohydrates''. When glucose is not supplied in the diet and glycogen stores have been depleted, animals may also synthesize glucose in the [[liver]] and (to a lesser extent) in the [[kidney]]s from non-carbohydrate intermediates. [[Lactate]] from active skeletal muscle, [[amino acids]] from protein in the diet or protein in muscle, | + | *''The synthesis of glucose from non-carbohydrates''. When glucose is not supplied in the diet and glycogen stores have been depleted, animals may also synthesize glucose in the [[liver]] and (to a lesser extent) in the [[kidney]]s from non-carbohydrate intermediates. [[Lactate]] from active skeletal muscle, [[amino acids]] from protein in the diet or protein in muscle, and [[glycerol]], derived from the [[hydrolysis]] of ''fats'', may contribute to the synthesis of glucose by [[gluconeogenesis]]. |
==The functions of glucose== | ==The functions of glucose== | ||
| − | + | Before the cell can utilize glucose, an enzyme chemically modifies glucose by the addition of a phosphate group ([[phosphorylation]]). The cell may not contain glucose at a higher concentration than the one present in the bloodstream. Since the [[cell membrane]] is impermeable to this modified form, called ''glucose-6-phosphate'', this process effectively "traps" glucose inside the cell, allowing the recovery of more glucose from the bloodstream. Glucose-6-phosphate, in turn, can be used for three major functions, depending on the specific conditions within the cell and the overall needs of the organism: | |
===Glucose is a major energy source=== | ===Glucose is a major energy source=== | ||
| − | Glucose is a ubiquitous fuel in [[biology]]. When chemical energy | + | Glucose is a ubiquitous fuel in [[biology]]. When chemical energy is needed, glucose is oxidized to [[pyruvate]] through a process known as [[glycolysis]], which is the energy source for certain organisms called [[obligate anaerobes]] that cannot utilize oxygen for metabolism. In [[aerobic]] organisms, however, the pyruvate typically continues onward to the reactions of the [[Citric acid cycle]] (TCAC) and the [[electron transport chain]], forming [[carbon dioxide|CO<sub>2</sub>]] and [[water]]. These later reactions generate about 18 times more energy than glycolysis, mostly in the form of [[adenosine triphosphate|ATP]]. |
===Glucose plays a role in the synthesis of non-carbohydrates=== | ===Glucose plays a role in the synthesis of non-carbohydrates=== | ||
| − | Glucose and its metabolites can also be mobilized when carbon skeletons are needed | + | Glucose and its metabolites can also be mobilized when carbon skeletons are needed. That is, glucose also participates in the synthesis of complex molecules ([[anabolism]]) in addition to its role in the [[catabolic]] pathways that break down molecules into smaller components. For example, glucose-6-phosphate can enter the [[pentose phosphate pathway]], which generates the five-carbon ([[pentose]]) sugar [[ribose]] for the synthesis of [[nucleotides]], the building blocks of the [[nucleic acids]] [[DNA]] and [[RNA]]. |
| − | In plants and most animals, glucose is a precursor for the production of [[vitamin C]] (ascorbic acid). | + | In plants and most animals (excepting [[guinea pigs]] and [[primates]], such as humans), glucose is a precursor for the production of [[vitamin C]] (ascorbic acid). [[Polymers]] of glucose may also be bound to proteins (to form [[glycoproteins]]) or lipids (to form [[glycolipids]]). The addition of sugar chains may function to assist proteins in folding into their characteristic three-dimensional structure, to enhance the stability of proteins and membrane lipids, or to act as recognition sites for specific chemicals. |
| − | ===Glucose is a component of | + | ===Glucose is a component of other carbohydrates=== |
| − | When the organism has an abundant supply of both ATP and glucose, | + | When the organism has an abundant supply of both ATP and glucose, glycogen, starch, and [[cellulose]], the common glucose [[polymer]]s ([[polysaccharide]]s), can be synthesized. While glycogen and starch serve as energy-storage molecules, cellulose plays a primarily structural role in green plants. |
| − | ==The | + | ==The regulation of blood glucose== |
| − | + | Given the importance of glucose as the preferred fuel of the brain, mammals must maintain a constant blood glucose level (which typically falls between 4.4 mM and 6.7 mM in an adult male human) for health and survival. | |
| − | The concentration of blood glucose is regulated through the action of | + | The concentration of blood glucose is mainly regulated through the action of [[hormones]]. Specifically, the hormone [[insulin]] directs the flow of glucose from the blood into liver, muscle, and [[adipose]] (fat storage) cells. It also promotes the increased synthesis of glycogen when energy needs have been met (for example, after a meal). When blood glucose levels fall (e.g., several hours after a meal), [[glucagon]] and [[epinephrine]] (also known as ''adrenaline'') retrieve glucose from its storage form as glycogen in liver and muscle tissue. Low levels of insulin in this state also mean that the entry of glucose into muscle and adipose cells decrease, so that these cells switch to the use of non-carbohydrate fuels. |
| + | |||
| + | The liver is a major control site of blood glucose levels, with the ability to respond to hormonal signals that indicate reduced or elevated blood glucose levels. One of the most important functions of the liver is to produce glucose for [[circulation]]. | ||
| − | + | Low blood glucose levels ([[hypoglycemia]]) can result in impaired functioning of the [[central nervous system]], which may manifest itself in dizziness, speech problems, or even loss of consciousness. | |
| − | + | [[Hyperglycemia]] (elevated blood sugar) indicates an overproduction of glucose by the liver cells accompanied by an inability of other cells to utilize glucose. Patients with [[Diabetes mellitus#Type 1|type 1 diabetes mellitus]] depend on external sources of insulin for their survival because their autoimmune system destroys the cells in the [[pancreas]] that secrete insulin; patients with the more common [[Diabetes mellitus#Type 2|type 2 diabetes mellitus]] may have relatively low insulin production or resistance to its effects. | |
==Commercial production== | ==Commercial production== | ||
Revision as of 01:58, 21 August 2006
| Glucose | |
|---|---|
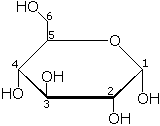
| |
| Chemical name | 6-(hydroxymethyl)oxane-2,3,4,5-tetrol |
| Synonym for D-glucose | dextrose |
| Varieties of D-glucose | α-D-glucose; β-D-glucose |
| Abbreviations | Glc |
| Chemical formula | C6H12O6 |
| Molecular mass | 180.16 g mol−1 |
| Melting point | α-D-glucose: 146°C β-D-glucose: 150°C |
| Density | 1.54 g cm-3 |
| CAS number | 50-99-7 (D-glucose) |
| CAS number | 921-60-8 (L-glucose) |
| SMILES | C(C1C(C(C(C(O1)O)O)O)O)O |
Glucose (Glc) is a monosaccharide (or simple sugar) with the chemical formula C6H12O6. It is the major free sugar circulating in the blood of higher animals, and the preferred fuel of the brain and nervous system, as well as red blood cells (erythrocytes).
As a universal substrate for the production of cellular energy, glucose is of central importance in the metabolism of all life forms. It is one of the main products of photosynthesis, the process by which photoautotrophs such as plants and algae convert energy from sunlight into chemical energy to be used by the cell. Glucose is also a major starting point for cellular respiration, in which the chemical bonds of energy-rich molecules such as glucose are converted into energy usable for life processes.
All major dietary carbohydrates contain glucose, either as their only building block, as in starch and glycogen, or in combination with another monosaccharide, as in sucrose (“table sugar”) and lactose, the primary sugar found in milk.
The natural form of glucose (D-glucose) is also referred to as dextrose, especially in the food industry.
The structure of glucose
Sugars are classified according to two properties: (1) number of carbon atoms and (2) type of functional group (either an aldehyde or a ketone group). Glucose, which has six carbon atoms (i.e., it is a hexose sugar) and contains an aldehyde group (-CHO), is thus referred to as an aldohexose.
The glucose molecule can exist in an open-chain (acyclic) form and a ring (cyclic) form. In solution and at neutral pH, the cyclic form is predominant at equilibrium. When glucose exists in cyclic form, the functional group is not free, making the molecule less reactive. This preference for the less reactive ring form offers a possible explanation for the crucial and widespread use of glucose in metabolism, as opposed to another monosaccharide such as fructose (Fru). The low tendency of glucose, in comparison to other hexose sugars, to non-specifically react with the amino groups of proteins might explain its importance to advanced life.
Isomers
Glucose has 4 optic centers which means that in theory glucose can have (4²-1) = 15 optical stereoisomers. Only 7 of these are found in living organisms, and of these galactose (Gal) and mannose (Man) are the most important. These eight isomers (including glucose itself) are all diastereoisomers in relation to each other (i.e., they are not mirror images), and all belong to the D-series.
Natural sources of glucose
- The conversion of light energy into chemical energy. Glucose is one of the products of photosynthesis in plants and algae, as well as some bacteria and protists.
- The breakdown of storage forms of glucose. Glucose can be obtained through the breakdown of glycogen, the storage form of glucose in animals and fungi, through a process known as glycogenolysis. Glycogen is an auxiliary energy source, tapped and converted back into glucose when there is need for energy. In plants, glucose is stored as starch.
- The synthesis of glucose from non-carbohydrates. When glucose is not supplied in the diet and glycogen stores have been depleted, animals may also synthesize glucose in the liver and (to a lesser extent) in the kidneys from non-carbohydrate intermediates. Lactate from active skeletal muscle, amino acids from protein in the diet or protein in muscle, and glycerol, derived from the hydrolysis of fats, may contribute to the synthesis of glucose by gluconeogenesis.
The functions of glucose
Before the cell can utilize glucose, an enzyme chemically modifies glucose by the addition of a phosphate group (phosphorylation). The cell may not contain glucose at a higher concentration than the one present in the bloodstream. Since the cell membrane is impermeable to this modified form, called glucose-6-phosphate, this process effectively "traps" glucose inside the cell, allowing the recovery of more glucose from the bloodstream. Glucose-6-phosphate, in turn, can be used for three major functions, depending on the specific conditions within the cell and the overall needs of the organism:
Glucose is a major energy source
Glucose is a ubiquitous fuel in biology. When chemical energy is needed, glucose is oxidized to pyruvate through a process known as glycolysis, which is the energy source for certain organisms called obligate anaerobes that cannot utilize oxygen for metabolism. In aerobic organisms, however, the pyruvate typically continues onward to the reactions of the Citric acid cycle (TCAC) and the electron transport chain, forming CO2 and water. These later reactions generate about 18 times more energy than glycolysis, mostly in the form of ATP.
Glucose plays a role in the synthesis of non-carbohydrates
Glucose and its metabolites can also be mobilized when carbon skeletons are needed. That is, glucose also participates in the synthesis of complex molecules (anabolism) in addition to its role in the catabolic pathways that break down molecules into smaller components. For example, glucose-6-phosphate can enter the pentose phosphate pathway, which generates the five-carbon (pentose) sugar ribose for the synthesis of nucleotides, the building blocks of the nucleic acids DNA and RNA.
In plants and most animals (excepting guinea pigs and primates, such as humans), glucose is a precursor for the production of vitamin C (ascorbic acid). Polymers of glucose may also be bound to proteins (to form glycoproteins) or lipids (to form glycolipids). The addition of sugar chains may function to assist proteins in folding into their characteristic three-dimensional structure, to enhance the stability of proteins and membrane lipids, or to act as recognition sites for specific chemicals.
Glucose is a component of other carbohydrates
When the organism has an abundant supply of both ATP and glucose, glycogen, starch, and cellulose, the common glucose polymers (polysaccharides), can be synthesized. While glycogen and starch serve as energy-storage molecules, cellulose plays a primarily structural role in green plants.
The regulation of blood glucose
Given the importance of glucose as the preferred fuel of the brain, mammals must maintain a constant blood glucose level (which typically falls between 4.4 mM and 6.7 mM in an adult male human) for health and survival.
The concentration of blood glucose is mainly regulated through the action of hormones. Specifically, the hormone insulin directs the flow of glucose from the blood into liver, muscle, and adipose (fat storage) cells. It also promotes the increased synthesis of glycogen when energy needs have been met (for example, after a meal). When blood glucose levels fall (e.g., several hours after a meal), glucagon and epinephrine (also known as adrenaline) retrieve glucose from its storage form as glycogen in liver and muscle tissue. Low levels of insulin in this state also mean that the entry of glucose into muscle and adipose cells decrease, so that these cells switch to the use of non-carbohydrate fuels.
The liver is a major control site of blood glucose levels, with the ability to respond to hormonal signals that indicate reduced or elevated blood glucose levels. One of the most important functions of the liver is to produce glucose for circulation.
Low blood glucose levels (hypoglycemia) can result in impaired functioning of the central nervous system, which may manifest itself in dizziness, speech problems, or even loss of consciousness.
Hyperglycemia (elevated blood sugar) indicates an overproduction of glucose by the liver cells accompanied by an inability of other cells to utilize glucose. Patients with type 1 diabetes mellitus depend on external sources of insulin for their survival because their autoimmune system destroys the cells in the pancreas that secrete insulin; patients with the more common type 2 diabetes mellitus may have relatively low insulin production or resistance to its effects.
Commercial production
Glucose is produced commercially through the breakdown of starch in an enzyme-catalyzed process called hydrolysis (a chemical reaction in which a molecule is split into two parts through the addition of water). The enzymatic process has two stages:
- Over the course of 1-2 hours near 100 °C, enzymes break the starch into smaller carbohydrates containing on average 5-10 glucose units each. Some variations on this process briefly heat the starch mixture to 130 °C or hotter one or more times. This heat treatment improves the solubility of starch in water, but deactivates the enzyme, so that fresh enzyme must be added to the mixture after each heating.
- In the second step, known as saccharification, the partially hydrolyzed starch is completely hydrolyzed to glucose using the glucoamylase enzyme from the fungus Aspergillus niger. Typical reaction conditions are pH 4.0–4.5, 60 °C, and a carbohydrate concentration of 30–35% by weight. Under these conditions, starch can be converted to glucose at 96% yield after 1–4 days. Still higher yields can be obtained using more dilute solutions, but this approach requires larger reactors and the processing of a greater volume of water, which is not generally economical.
The resulting glucose solution is then purified by filtration and concentrated in a multiple-effect evaporator. Solid D-glucose is finally produced by repeated crystallizations.
Many crops can be used as sources of starch in the commerical production of glucose, including maize, rice, wheat, potato, cassava, arrowroot, and sago. In the United States, cornstarch (from maize) is used almost exclusively.
ReferencesISBN links support NWE through referral fees
- Stryer, Lubert. 1995. Biochemistry, 4th edition. New York, NY: W.H. Freeman.
External links
- EINECS number 200-075-1 (D-glucose)
- EINECS number 213-068-3 (L-glucose)
- CID 5793 from PubChem (D-glucose)
- CID 206 from PubChem (L-glucose)
- More on the chemistry and function of glucose in biology at EvoWiki
- Computational Chemistry Wiki
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.