Difference between revisions of "Radioactive decay" - New World Encyclopedia
| Line 88: | Line 88: | ||
Radioactive decay results in a reduction of summed rest [[mass]], which is [[Mass in special relativity|converted to energy]] (the ''disintegration energy'') according to the formula <math>E = mc^2</math>. This energy is released as kinetic energy of the emitted particles. The energy remains associated with a measure of mass of the decay system [[invariant mass]], inasmuch the kinetic energy of emitted particles contributes also to the total invariant mass of systems. Thus, the sum of rest masses of particles is not conserved in decay, but the ''system'' mass or system invariant mass (as also system total energy) is conserved. | Radioactive decay results in a reduction of summed rest [[mass]], which is [[Mass in special relativity|converted to energy]] (the ''disintegration energy'') according to the formula <math>E = mc^2</math>. This energy is released as kinetic energy of the emitted particles. The energy remains associated with a measure of mass of the decay system [[invariant mass]], inasmuch the kinetic energy of emitted particles contributes also to the total invariant mass of systems. Thus, the sum of rest masses of particles is not conserved in decay, but the ''system'' mass or system invariant mass (as also system total energy) is conserved. | ||
| − | == | + | == Radioactive Series == |
| + | In a simple radioactive decay, the new nucleus is stable. C-14 and K-40 | ||
| + | |||
| + | |||
The daughter nuclide of a decay event is usually also unstable, sometimes even more unstable than the parent. If this is the case, it will proceed to decay again. A sequence of several decay events, producing in the end a stable nuclide, is a ''[[decay chain]]''. | The daughter nuclide of a decay event is usually also unstable, sometimes even more unstable than the parent. If this is the case, it will proceed to decay again. A sequence of several decay events, producing in the end a stable nuclide, is a ''[[decay chain]]''. | ||
Revision as of 17:27, 21 October 2007
- "Radioactive" and "Radioactivity" redirect here..
Radioactive decay is the process in which an unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. This decay, or loss of energy, results in an atom of one type, called the parent nuclide transforming to an atom of a different type, called the daughter nuclide. For example: a carbon-14 atom (the "parent") emits radiation and transforms to a nitrogen-14 atom (the "daughter.") This transformation involves quantum probability, so it is impossible to predict when a particular atom will decay. Given a large number of atoms, however, the decay rate is predictable and measured by the "half-life"—the time it takes for 50% of the atoms to undergo the change. The half-life of radioactive atoms varies enormously; from fractions of a millisecond to billions of years.
The SI unit of radioactive decay (the phenomenon of natural and artificial radioactivity) is the becquerel (Bq). One Bq is defined as one transformation (or decay) per second. Since any reasonably-sized sample of radioactive material contains many atoms, a Bq is a tiny measure of activity; amounts on the order of TBq (terabecquerel) or GBq (gigabecquerel) are commonly used. Another unit of (radio)activity is the curie, Ci, which was originally defined as the activity of one gram of pure radium, isotope Ra-226. At present it is equal (by definition) to the activity of any radionuclide decaying with a disintegration rate of 3.7 × 1010 Bq. The use of Ci is presently discouraged by SI.
Explanation
The neutrons and protons that constitute nuclei, as well as other particles that may approach them, are governed by several interactions. The strong nuclear force, not observed at the familiar macroscopic scale, is the most powerful force over subatomic distances. The electrostatic force is also significant, while the weak nuclear force is responsible for Beta decay.
The interplay of these forces is simple. Some configurations of the particles in a nucleus have the property that, should they shift ever so slightly, the particles could fall into a lower-energy arrangement (with the extra energy moving elsewhere). One might draw an analogy with a snowfield on a mountain: while friction between the snow crystals can support the snow's weight, the system is inherently unstable with regards to a lower-potential-energy state, and a disturbance may facilitate the path to a greater entropy state (i.e., towards the ground state where heat will be produced, and thus total energy is distributed over a larger number of quantum states). Thus, an avalanche results. The total energy does not change in this process, but because of entropy effects, avalanches only happen in one direction, and the end of this direction, which is dictated by the largest number of chance-mediated ways to distribute available energy, is what we commonly refer to as the "ground state."
Such a collapse (a decay event) requires a specific activation energy. In the case of a snow avalanche, this energy classically comes as a disturbance from outside the system, although such disturbances can be arbitrarily small. In the case of an excited atomic nucleus, the arbitrarily small disturbance comes from quantum vacuum fluctuations. A nucleus (or any excited system in quantum mechanics) is unstable, and can thus spontaneously stabilize to a less-excited system. This process is driven by entropy considerations: the energy does not change, but at the end of the process, the total energy is more diffused in spacial volume. The resulting transformation alters the structure of the nucleus. Such a reaction is thus a nuclear reaction, in contrast to chemical reactions, which also are driven by entropy, but which involve changes in the arrangement of the outer electrons of atoms, rather than their nuclei.
Some nuclear reactions do involve external sources of energy, in the form of collisions with outside particles. However, these are not considered decay. Rather, they are examples of induced nuclear reactions. Nuclear fission and fusion are common types of induced nuclear reactions.
Discovery
Radioactivity was first discovered in 1896 by the French scientist Henri Becquerel while working on phosphorescent materials. These materials glow in the dark after exposure to light, and he thought that the glow produced in cathode ray tubes by X-rays might somehow be connected with phosphorescence. So he tried wrapping a photographic plate in black paper and placing various phosphorescent minerals on it. All results were negative until he tried using uranium salts. The result with these compounds was a deep blackening of the plate.
However, it soon became clear that the blackening of the plate had nothing to do with phosphorescence because the plate blackened when the mineral was kept in the dark. Also non-phosphorescent salts of uranium and even metallic uranium blackened the plate. Clearly there was some new form of radiation that could pass through paper that was causing the plate to blacken.
At first it seemed that the new radiation was similar to the then recently discovered X-rays. However further research by Becquerel, Marie Curie, Pierre Curie, Ernest Rutherford and others discovered that radioactivity was significantly more complicated. Different types of decay can occur, but Rutherford was the first to realize that they all occur with the same mathematical approximately exponential formula (see below).
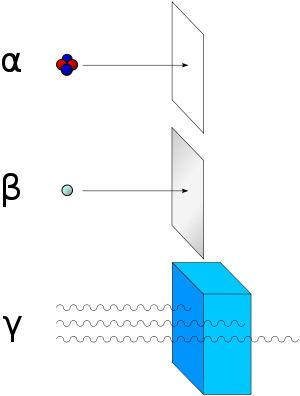
As for types of radioactive radiation, it was found that an electric or magnetic field could split such emissions into three types of beams. For lack of better terms, the rays were given the alphabetic names alpha, beta, and gamma, names they still hold today. It was immediately obvious from the direction of electromagnetic forces that alpha rays carried a positive charge, beta rays carried a negative charge, and gamma rays were neutral. From the magnitude of deflection, it was also clear that alpha particles were much more massive than beta particles. Passing alpha rays through a thin glass membrane and trapping them in a discharge tube allowed researchers to study the emission spectrum of the resulting gas, and ultimately prove that alpha particles are in fact helium nuclei. Other experiments showed the similarity between beta radiation and cathode rays; they are both streams of electrons, and between gamma radiation and X-rays, which are both high energy electromagnetic radiation.
Although alpha, beta, and gamma are most common, other types of decay were eventually discovered. Shortly after discovery of the neutron in 1932, it was discovered by Enrico Fermi that certain rare decay reactions give rise to neutrons as a decay particle. Isolated proton emission was also eventually observed in some elements. Shortly after the discovery of the positron in cosmic ray products, it was realized that the same process that operates in classical beta decay can also produce positrons (positron emission), analogously to negative electrons. Each of the two types of beta decay acts to move a nucleus toward a ratio of neutrons and protons which has the least energy for the combination. Finally, in a phenomenon called cluster decay, specific combinations of neutrons and protons other than alpha particles were found to occasionally spontaneously be emitted from atoms.
Still other types of radioactive decay were found which emit previously seen particles, but by different mechanisms. An example is internal conversion, which results in electron and sometimes high energy photon emission, even though it involves neither beta nor gamma decay.
The early researchers also discovered that many other chemical elements besides uranium have radioactive isotopes. A systematic search for the total radioactivity in uranium ores also guided Marie Curie to isolate a new element polonium and to separate a new element radium from barium; the two elements' chemical similarity would otherwise have made them difficult to distinguish.
The dangers of radioactivity and of radiation were not immediately recognized. Acute effects of radiation were first observed in the use of X-rays when the Serbo-Croatian-American electric engineer Nikola Tesla intentionally subjected his fingers to X-rays in 1896. He published his observations concerning the burns that developed, though he attributed them to ozone rather than to the X-rays. Fortunately his injuries healed later.
The genetic effects of radiation, including the effects on cancer risk, were recognized much later. It was only in 1927 that Hermann Joseph Muller published his research that showed the genetic effects. In 1946 he was awarded the Nobel prize for his findings.
Before the biological effects of radiation were known, many physicians and corporations had begun marketing radioactive substances as patent medicine and Radioactive quackery; particularly alarming examples were radium enema treatments, and radium-containing waters to be drunk as tonics. Marie Curie spoke out against this sort of treatment, warning that the effects of radiation on the human body were not well understood (Curie later died from aplastic anemia assumed due to her own work with radium, but later examination of her bones showed that she had been a careful laboratory worker and had a low burden of radium; a better candidate for her disease was her long exposure to unshielded X-ray tubes while a volunteer medical worker in WW I). By the 1930s, after a number of cases of bone-necrosis and death in enthusiasts, radium-containing medical products had nearly vanished from the market.
Modes of decay
Radionuclides can undergo a number of different reactions. These are summarized in the following table. A nucleus with positive charge (atomic number) Z and atomic weight A is represented as (A, Z).
| Mode of decay | Participating particles | Daughter nucleus |
|---|---|---|
| Decays with emission of nucleons: | ||
| Alpha decay | An alpha particle (A=4, Z=2) emitted from nucleus | (A-4, Z-2) |
| Proton emission | A proton ejected from nucleus | (A-1, Z-1) |
| Neutron emission | A neutron ejected from nucleus | (A-1, Z) |
| Double proton emission | Two protons ejected from nucleus simultaneously | (A-2, Z-2) |
| Spontaneous fission | Nucleus disintegrates into two or more smaller nuclei and other particles | - |
| Cluster decay | Nucleus emits a specific type of smaller nucleus (A1, Z1) larger than an alpha particle | (A-A1, Z-Z1) + (A1,Z1) |
| Different modes of beta decay: | ||
| Beta-Negative decay | A nucleus emits an electron and an antineutrino | (A, Z+1) |
| Positron emission, also Beta-Positive decay | A nucleus emits a positron and a neutrino | (A, Z-1) |
| Electron capture | A nucleus captures an orbiting electron and emits a neutrino - The daughter nucleus is left in an excited and unstable state | (A, Z-1) |
| Double beta decay | A nucleus emits two electrons and two antineutrinos | (A, Z+2) |
| Double electron capture | A nucleus absorbs two orbital electrons and emits two neutrinos - The daughter nucleus is left in an excited and unstable state | (A, Z-2) |
| Electron capture with positron emission | A nucleus absorbs one orbital electron, emits one positron and two neutrinos | (A, Z-2) |
| Double positron emission | A nucleus emits two positrons and two neutrinos | (A, Z-2) |
| Transitions between states of the same nucleus: | ||
| Gamma decay | Excited nucleus releases a high-energy photon (gamma ray) | (A, Z) |
| Internal conversion | Excited nucleus transfers energy to an orbital electron and it is ejected from the atom | (A, Z) |
Radioactive decay results in a reduction of summed rest mass, which is converted to energy (the disintegration energy) according to the formula . This energy is released as kinetic energy of the emitted particles. The energy remains associated with a measure of mass of the decay system invariant mass, inasmuch the kinetic energy of emitted particles contributes also to the total invariant mass of systems. Thus, the sum of rest masses of particles is not conserved in decay, but the system mass or system invariant mass (as also system total energy) is conserved.
Radioactive Series
In a simple radioactive decay, the new nucleus is stable. C-14 and K-40
The daughter nuclide of a decay event is usually also unstable, sometimes even more unstable than the parent. If this is the case, it will proceed to decay again. A sequence of several decay events, producing in the end a stable nuclide, is a decay chain.
Many radionuclides have several different observed modes of decay. Bismuth-212, for example, has three. Thus a given nuclide may lead to several different decay chains.
Of the commonly occurring forms of radioactive decay, the only one that changes the number of aggregate protons and neutrons (nucleons) contained in the nucleus is alpha emission, which reduces it by four. Thus, the number of nucleons modulo 4 is preserved across any decay chain.
Occurrence and applications
According to the Big Bang theory, radioactive isotopes of the lightest elements (H, He, and traces of Li) were produced very shortly after the emergence of the universe. However, these nuclides are so highly unstable that virtually none of them have survived to today. Most radioactive nuclei are therefore relatively young, having formed in stars (particularly supernovae) and during ongoing interactions between stable isotopes and energetic particles. For example, carbon-14, a radioactive nuclide with a half-life of only 5730 years, is constantly produced in Earth's upper atmosphere due to interactions between cosmic rays and nitrogen.
Radioactive decay has been put to use in the technique of radioisotopic labeling, used to track the passage of a chemical substance through a complex system (such as a living organism). A sample of the substance is synthesized with a high concentration of unstable atoms. The presence of the substance in one or another part of the system is determined by detecting the locations of decay events.
On the premise that radioactive decay is truly random (rather than merely chaotic), it has been used in hardware random-number generators. Because the process is not thought to vary significantly in mechanism over time, it is also a valuable tool in estimating the absolute ages of certain materials. For geological materials, the radioisotopes and certain of their decay products become trapped when a rock solidifies, and can then later be used (subject to many well-known qualifications) to estimate the date of the solidification. These include checking the results of several simultaneous processes and their products against each other, within the same sample.
Radioactive decay rates
The decay rate, or activity, of a radioactive substance are characterized by:
Constant quantities:
- half life—symbol —the time for half of a substance to decay.
- mean lifetime—symbol —the average lifetime of any given particle.
- decay constant—symbol —the inverse of the mean lifetime.
- (Note that although these are constants, they are associated with statistically random behavior of substances, and predictions using these constants are less accurate for small number of atoms.)
Time-variable quantities:
- Total activity—symbol —number of decays an object undergoes per second.
- Number of particles—symbol —the total number of particles in the sample.
- Specific activity—symbol —number of decays per second per amount of substance. The "amount of substance" can be the unit of either mass or volume.)
These are related as follows:
-
- where
- is the initial amount of active substance—substance that has the same percentage of unstable particles as when the substance was formed.
- where
Activity measurements
The units in which activities are measured are: becquerel (symbol Bq) = number of disintegrations per second; curie (Ci) = 3.7 × 1010 disintegrations per second. Low activities are also measured in disintegrations per minute (dpm).
Decay timing
As discussed above, the decay of an unstable nucleus is entirely random and it is impossible to predict when a particular atom will decay. However, it is equally likely to decay at any time. Therefore, given a sample of a particular radioisotope, the number of decay events –dN expected to occur in a small interval of time dt is proportional to the number of atoms present. If N is the number of atoms, then the probability of decay (– dN/N) is proportional to dt:
Particular radionuclides decay at different rates, each having its own decay constant (λ). The negative sign indicates that N decreases with each decay event. The solution to this first-order differential equation is the following function:
This function represents exponential decay. It is only an approximate solution, for two reasons. Firstly, the exponential function is continuous, but the physical quantity N can only take non-negative integer values. Secondly, because it describes a random process, it is only statistically true. However, in most common cases, N is a very large number and the function is a good approximation.
In addition to the decay constant, radioactive decay is sometimes characterized by the mean lifetime. Each atom "lives" for a finite amount of time before it decays, and the mean lifetime is the arithmetic mean of all the atoms' lifetimes. It is represented by the symbol , and is related to the decay constant as follows:
A more commonly used parameter is the half-life. Given a sample of a particular radionuclide, the half-life is the time taken for half the radionuclide's atoms to decay. The half life is related to the decay constant as follows:
This relationship between the half-life and the decay constant shows that highly radioactive substances are quickly spent, while those that radiate weakly endure longer. Half-lives of known radionuclides vary widely, from more than 1019 years (such as for very nearly stable nuclides, e.g. 209Bi), to 10-23 seconds for highly unstable ones.
See also
- Alpha particle
- Nuclear physics
- Radiation
- Radiation therapy
- Radiometric dating
- Inner transition metal
- Half-life
ReferencesISBN links support NWE through referral fees
- Krane, Kenneth S., and David Halliday. 1988. Introductory Nuclear Physics. New York: Wiley. ISBN 047180553X.
- Martin, Brian. 2006. Nuclear and Particle Physics: An Introduction. Hoboken, NJ: Wiley. ISBN 0470025328.
- Poenaru, D. N. 1996. Nuclear Decay Modes. Fundamental and Applied Nuclear Physics Series. Philadelphia: Institute of Physics. ISBN 0750303387.
- Saling, James. 2001. Radioactive Waste Management. New York, NY: Taylor & Francis. ISBN 1560328428.
- Seiden, Abraham. 2004. Particle Physics: A Comprehensive Introduction. San Francisco, CA: Addison Wesley. ISBN 0805387366.
- Tipler, Paul, and Ralph Llewellyn. 2002. Modern Physics. 4th ed. New York, NY: W.H. Freeman. ISBN 0-7167-4345-0.
- Turner, James E. 1995. Atoms, Radiation, and Radiation Protection. 2nd ed. New York: Wiley. ISBN 0471595810.
External links
- General information. Retrieved October 14, 2007.
- Nomenclature of nuclear chemistry. Retrieved October 14, 2007.
- Some theoretical questions of nuclear stability. Retrieved October 14, 2007.
- Decay heat rate|quantity calculation. Retrieved October 14, 2007.
- Specific activity and related topics. Retrieved October 14, 2007.
- The Lund/LBNL Nuclear Data Search - Contains tabulated information on radioactive decay types and energies. Retrieved October 14, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.