Difference between revisions of "Pyridine" - New World Encyclopedia
(imported latest version of article from Wikipedia) |
|||
| Line 1: | Line 1: | ||
| + | {{Claimed}} | ||
{{Chembox new | {{Chembox new | ||
| Name = Pyridine | | Name = Pyridine | ||
| Line 30: | Line 31: | ||
| OtherCpds = [[Aniline]]<br />[[Pyrimidine]]}} | | OtherCpds = [[Aniline]]<br />[[Pyrimidine]]}} | ||
}} | }} | ||
| − | '''Pyridine''' is a [[chemical compound]] with the formula [[Carbon|C<sub>5</sub>]][[Hydrogen|H<sub>5</sub>]][[Nitrogen|N]]. It is a liquid with a distinctively putrid, fishy odour. Pyridine is a simple and fundamentally important [[heterocyclic]] [[aromatic]] [[organic compound]] that is structurally related to [[benzene]], wherein one CH group in the six-membered ring is replaced by a | + | '''Pyridine''' is a [[chemical compound]] with the formula [[Carbon|C<sub>5</sub>]][[Hydrogen|H<sub>5</sub>]][[Nitrogen|N]]. It is a liquid with a distinctively putrid, fishy odour. Pyridine is a simple and fundamentally important [[heterocyclic]] [[aromatic]] [[organic compound]] that is structurally related to [[benzene]], wherein one CH group in the six-membered ring is replaced by a nitrogen atom. The pyridine ring occurs in many important compounds, including the [[nicotinamide]]s. Pyridine is sometimes used as a [[ligand]] in [[coordination chemistry]]. As a ligand, it is usually abbreviated '''py'''. |
==Basicity== | ==Basicity== | ||
| Line 62: | Line 63: | ||
==Safety and Environmental== | ==Safety and Environmental== | ||
| − | Pyridine is toxic with LD<sub>50</sub> in rats (oral) of 891 mg kg<sup>–1</sup>. It is volatile and can be absorbed through skin. Available data indicate that "''exposure to pyridine in drinking-water led to reduction of sperm motility at all dose levels in mice and increased estrous cycle length at the highest dose level in rats''".<ref name="IARC1">{{cite web | last = International Agency for Research on Cancer (IARC) | authorlink = International Agency for Research on Cancer | title = Pyridine Summary & Evaluation | work = IARC Summaries & Evaluations | publisher = IPCS INCHEM | date = | + | Pyridine is toxic with LD<sub>50</sub> in rats (oral) of 891 mg kg<sup>–1</sup>. It is volatile and can be absorbed through skin. Available data indicate that "''exposure to pyridine in drinking-water led to reduction of sperm motility at all dose levels in mice and increased estrous cycle length at the highest dose level in rats''".<ref name="IARC1">{{cite web | last = International Agency for Research on Cancer (IARC) | authorlink = International Agency for Research on Cancer | title = Pyridine Summary & Evaluation | work = IARC Summaries & Evaluations | publisher = IPCS INCHEM | date = 2000-08-22 | url = http://www.inchem.org/documents/iarc/vol77/77-16.html | format = [[HTML]] | accessdate = 2007-01-17 }}</ref> Currently its evaluations as a possible [[carcinogenic]] agent showed there is inadequate evidence in humans for the carcinogenicity of pyridine, albeit there is limited evidence of carcinogenic effects on animals.<ref name="IARC1"/> Effects of an acute pyridine intoxication include dizziness, headache, nausea and anorexia. Further symptoms include abdominal pain and pulmonary congestion.<ref name="IARC1"/> Though resistant to oxidation, pyridine is readily degraded by bacteria, releasing ammonium and carbon dioxide as terminal degradation products.<ref>{{cite journal | author = Sims, G.K. and O'Loughlin, E.J. | title = Degradation of pyridines in the environment | journal = CRC Critical Reviews in Environmental Control | date = 1989 | volume = 19 | issue = 4 | pages = 309-340}}</ref> |
==Related compounds== | ==Related compounds== | ||
| Line 79: | Line 80: | ||
==See also== | ==See also== | ||
| + | |||
| + | * [[Benzene]] | ||
| + | * [[Pyrimidine]] | ||
* [[Simple aromatic ring]]s | * [[Simple aromatic ring]]s | ||
| − | + | ||
| + | == Notes == | ||
| + | <references/> | ||
==References== | ==References== | ||
| − | |||
| − | + | * McMurry, John. 2004. ''Organic Chemistry''. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052. | |
| + | |||
| + | * Morrison, Robert T., and Robert N. Boyd. 1992. ''Organic Chemistry''. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2. | ||
| + | |||
| + | * Solomons, T.W. Graham, and Fryhle, Craig B. 2004. ''Organic Chemistry''. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998. | ||
==External links== | ==External links== | ||
| Line 96: | Line 105: | ||
{{Functional Groups}} | {{Functional Groups}} | ||
| − | [[Category: | + | [[Category:Physical sciences]] |
| − | [[Category: | + | [[Category:Chemistry]] |
| − | [[Category: | + | [[Category:Organic chemistry]] |
| − | |||
| − | |||
| − | |||
| − | + | {{credit|166553158}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Revision as of 16:18, 1 November 2007
| Pyridine | |
|---|---|
| |
| IUPAC name | Pyridine |
| Other names | Azabenzene Azine py |
| Identifiers | |
| CAS number | [] |
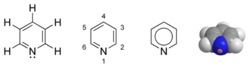
| SMILES | C1=NC=CC=C1 |
| Properties | |
| Molecular formula | C5H5N |
| Appearance | colourless liquid |
| Density | 0.9819 g/cm³, liquid |
| Melting point |
−41.6 °C |
| Boiling point |
115.2 °C |
| Solubility in water | Miscible |
| Viscosity | 0.94 cP at 20 °C |
| Hazards | |
| EU classification | Flammable (F) Harmful (Xn) |
| NFPA 704 |
|
| Flash point | 21 °C |
| Related Compounds | |
| Related amines | Picoline Quinoline |
| Related compounds | Aniline Pyrimidine |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
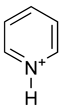
Pyridine is a chemical compound with the formula C5H5N. It is a liquid with a distinctively putrid, fishy odour. Pyridine is a simple and fundamentally important heterocyclic aromatic organic compound that is structurally related to benzene, wherein one CH group in the six-membered ring is replaced by a nitrogen atom. The pyridine ring occurs in many important compounds, including the nicotinamides. Pyridine is sometimes used as a ligand in coordination chemistry. As a ligand, it is usually abbreviated py.
Basicity
Pyridine has a lone pair of electrons at the nitrogen atom. Because this lone pair is not delocalized into the aromatic pi-system, pyridine is basic with chemical properties similar to tertiary amines. The pKa of the conjugate acid is 5.30. Pyridine is protonated by reaction with acids and forms a positively charged aromatic polyatomic ion called pyridinium cation. The bond lengths and bond angles in pyridine and the pyridinium ion are almost identical[1] because protonation does not affect the aromatic pi system.
Pyridine as a solvent
Pyridine is widely used as a versatile solvent, since it is polar but aprotic. It is fully miscible with a very broad range of solvents including hexane and water. Deuterated pyridine, called pyridine-d5, is a common solvent for1H NMR spectroscopy.
Role in chemical synthesis
Pyridine is important in industrial organic chemistry, both as a fundamental building block and as a solvent and reagent in organic synthesis.[2] It is used as a solvent in Knoevenagel condensations.
Pyridine-borane, C5H5NBH3 (m.p. 10–11 °C) is a mild reducing agent with improved stability vs NaBH4 in protic solvents and improved solubility in aprotic organic solvents. Pyridine-sulfur trioxide, C5H5NSO3 (mp 175 °C) is a sulfonation agent used to convert alcohols to sulfonates, which in turn undergo C-O bond scission upon reduction with hydride agents.
It is also a starting material in the synthesis of compounds used as an intermediate in making insecticides, herbicides, pharmaceuticals, food flavorings, dyes, rubber chemicals, adhesives, paints, explosives and disinfectants. Pyridine is also used as a denaturant for antifreeze mixtures, for ethyl alcohol, and for fungicides, and as a dyeing aid for textiles.
Preparation and occurrence
Many methods exist in industry and in the laboratory (some of them named reactions) for the synthesis of pyridine and its derivatives:[3] Pyridine was originally isolated industrially from crude coal tar. It is currently synthesized from acetaldehyde, formaldehyde and ammonia, a process that involves the intermediacy of acrolein:
- CH2O + NH3 + 2 CH3CHO → C5H5N + 3 H2O
By substituting other aldehydes for acetaldehyde, one obtains alkyl and aryl substituted pyridines. 26,000 tons were produced worldwide in 1989.[4]
- The Hantzsch pyridine synthesis is a multicomponent reaction involving formaldehyde, a keto-ester and a nitrogen donor.
- Other examples of the pyridine class can be formed by the reaction of 1,5-diketones with ammonium acetate in acetic acid followed by oxidation. This reaction is called the "Kröhnke pyridine synthesis."
- Pyridium salts can be obtained in the Zincke reaction.
- The "Ciamician-Dennstedt Rearrangement" (1881) is the ring-expansion of pyrrole with dichlorocarbene to 3-chloropyridine and HCl[5]
- In the "Chichibabin pyridine synthesis" (Aleksei Chichibabin, 1906) the reactants are three equivalents of a linear aldehyde and ammonia
Organic reactions
In organic reactions pyridine behaves both as a tertiary amine with protonation, alkylation, acylation and N-oxidation at nitrogen and as an aromatic compound with Nucleophilic substitutions.
- Pyridine is a good nucleophile with a donor number of 33.1. It is easily attacked by alkylating agents to give N-alkylpyridinium salts.
- Nucleophilic aromatic substitution takes place at C2 and C4 for example in the Chichibabin reaction of pyridine with sodium amide to 2-aminopyridine. In the Emmert reaction (B. Emmert, 1939) pyridine is reacted with a ketone in presence of aluminium or magnesium and mercuric chloride to the carbinol also at C2[6].
Safety and Environmental
Pyridine is toxic with LD50 in rats (oral) of 891 mg kg–1. It is volatile and can be absorbed through skin. Available data indicate that "exposure to pyridine in drinking-water led to reduction of sperm motility at all dose levels in mice and increased estrous cycle length at the highest dose level in rats".[7] Currently its evaluations as a possible carcinogenic agent showed there is inadequate evidence in humans for the carcinogenicity of pyridine, albeit there is limited evidence of carcinogenic effects on animals.[7] Effects of an acute pyridine intoxication include dizziness, headache, nausea and anorexia. Further symptoms include abdominal pain and pulmonary congestion.[7] Though resistant to oxidation, pyridine is readily degraded by bacteria, releasing ammonium and carbon dioxide as terminal degradation products.[8]
Related compounds
Structurally or chemically related compounds are
- DMAP is short for 4-dimethylaminopyridine
- Bipyridine and viologen are simple polypyridine compounds consisting of two pyridine molecules joined by a single bond
- Terpyridine, a molecule of three pyridine rings connected together by two single bonds.
- Quinoline and Isoquinoline have pyridine and a benzene ring fused together.
- Aniline is a benzene derivative with an attached NH2 group and NOT a pyridine
- Diazines are compounds with one more carbon replaced by nitrogen such as Pyrazine and Pyramidine
- Triazines are compounds with two more carbons replaced by nitrogen and a tetrazine has four nitrogen atoms
- 2,6-Lutidine is a trivial name for 2,6-dimethylpyridine.
- Collidine is the trivial name for 2,4,6-trimethylpyridine.
- Pyridinium p-toluenesulfonate (PPTS) is a salt formed by proton exchange between pyridine and p-toluenesulfonic acid
- 2-Chloropyridine is a toxic environmentally significant component of the breakdown of the pesticide imidacloprid.
See also
- Benzene
- Pyrimidine
- Simple aromatic rings
Notes
- ↑ T. M. Krygowski, H. Szatyowicz, and J. E. Zachara J. Org. Chem. 2005 70(22) 8859 - 8865; Digital object identifier (DOI): 10.1021/jo051354h .
- ↑ Sherman, A. R. “Pyridine” in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. DOI:10.1002/047084289.
- ↑ Gilchrist, T.L. (1997). Heterocyclic Chemistry ISBN 0470204818
- ↑ Shinkichi Shimizu, Nanao Watanabe, Toshiaki Kataoka, Takayuki Shoji, Nobuyuki Abe, Sinji Morishita, Hisao Ichimura "Pyridine and Pyridine Derivatives" in "Ullmann's Encyclopedia of Industrial Chemistry" 2007; John Wiley & Sons: New York.
- ↑ Ciamician-Dennstedt Rearrangement @ drugfuture.com Link
- ↑ Histamine Antagonists. Basically Substituted Pyridine Derivatives Charles H. Tilford, Robert S. Shelton, and M. G. van Campen J. Am. Chem. Soc.; 1948; 70(12) pp 4001 - 4009; DOI:10.1021/ja01192a010
- ↑ 7.0 7.1 7.2 International Agency for Research on Cancer (IARC) (2000-08-22). Pyridine Summary & Evaluation (HTML). IARC Summaries & Evaluations. IPCS INCHEM. Retrieved 2007-01-17.
- ↑ Sims, G.K. and O'Loughlin, E.J. (1989). Degradation of pyridines in the environment. CRC Critical Reviews in Environmental Control 19 (4): 309-340.
ReferencesISBN links support NWE through referral fees
- McMurry, John. 2004. Organic Chemistry. 6th ed. Belmont, CA: Brooks/Cole. ISBN 0534420052.
- Morrison, Robert T., and Robert N. Boyd. 1992. Organic Chemistry. 6th ed. Englewood Cliffs, NJ: Prentice Hall. ISBN 0-13-643669-2.
- Solomons, T.W. Graham, and Fryhle, Craig B. 2004. Organic Chemistry. 8th ed. Hoboken, NJ: John Wiley. ISBN 0471417998.
External links
- International Chemical Safety Card 0323
- NIOSH Pocket Guide to Chemical Hazards
- Computational Chemistry Wiki
- Examples of Pyridines
- Synthesis of pyridines (overview of recent methods)
| Functional groups |
|---|
| Chemical class: Alcohol • Aldehyde • Alkane • Alkene • Alkyne • Amide • Amine • Azo compound • Benzene derivative • Carboxylic acid • Cyanate • Ester • Ether • Haloalkane • Imine • Isocyanide • Isocyanate • Ketone • Nitrile • Nitro compound • Nitroso compound • Peroxide • Phosphoric acid • Pyridine derivative • Sulfone • Sulfonic acid • Sulfoxide • Thioether • Thiol • Toluene derivative |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.