Difference between revisions of "Ozone" - New World Encyclopedia
(imported article from Wikipedia) |
Rosie Tanabe (talk | contribs) |
||
| (41 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Copyedited}}{{Paid}}{{Submitted}}{{Images OK}}{{Approved}} | ||
| + | [[Category:Public]] | ||
{| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | {| class="toccolours" border="1" style="float: right; clear: right; margin: 0 0 1em 1em; border-collapse: collapse;" | ||
! {{chembox header}} | {{PAGENAME}} | ! {{chembox header}} | {{PAGENAME}} | ||
| Line 69: | Line 71: | ||
| [[Flash point]],<br/>[[RTECS|RTECS number]], etc. | | [[Flash point]],<br/>[[RTECS|RTECS number]], etc. | ||
|- | |- | ||
| − | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br> materials in their | + | | {{chembox header}} | <small>Except where noted otherwise, data are given for<br> materials in their standard state (at 25° C, 100 kPa pressure)</small> |
|- | |- | ||
|} | |} | ||
| − | |||
| − | |||
| − | Ozone is a | + | '''Ozone''' (molecular formula O<sub>3</sub>) is a minor constituent of the [[Earth's atmosphere]], but its effects are highly significant. It is chemically very reactive and is involved in reactions that drive many of the chemical changes that occur in the atmosphere by day and by night. |
| + | {{toc}} | ||
| + | About 90 percent of the ozone in our atmosphere is contained in the [[Earth’s_atmosphere#Stratosphere|stratosphere]] (part of the upper atmosphere), and about 10 percent is contained in the [[Earth’s_atmosphere#Troposphere|troposphere]] (lower atmosphere). Ground-level ozone is an air [[pollutant]] with harmful effects on our respiratory system. On the other hand, ozone in the upper atmosphere protects living organisms by preventing damaging [[ultraviolet]] light from reaching the Earth's surface. | ||
| − | : | + | ==Discovery and notable characteristics == |
| + | Ozone was discovered in 1840 by [[Christian Friedrich Schönbein]], who named it after the [[Greek language|Greek]] word for smell (''ozein''), associating it with the peculiar odor in the air after [[lightning]] storms. [http://www.todayinsci.com/cgi-bin/indexpage.pl?http://www.todayinsci.com/8/8_29.htm]. The odor from a lightning strike, however, is from electrons freed during rapid chemical changes, not from the ozone itself [http://gcmd.gsfc.nasa.gov/Resources/FAQs/ozone.html]. | ||
| − | + | Each [[molecule]] of ozone consists of three oxygen [[atom]]s, and its molecular formula is therefore written as O<sub>3</sub>. As such, it is an [[allotrope]] of [[oxygen]] (dioxygen, O<sub>2</sub>), which is a much more stable and abundant gas. | |
| − | + | At standard temperature and pressure (0°C and 100 kilopascals pressure), ozone is a pale blue [[gas]]. It forms a dark blue [[liquid]] below −112° C and a dark blue [[solid]] below −193° C. It is a powerful [[oxidizing]] agent (see [[#Reactions|Reactions]] below). | |
| − | + | Ozone is unstable, and when it breaks down, it gives rise to ordinary oxygen (O<sub>2</sub>) and [[free radical]]s of atomic oxygen (O). The reaction is as follows. | |
| + | :O<sub>3</sub> → O<sub>2</sub> + O | ||
| + | The free radicals are highly reactive and damage or destroy most organic molecules. They can also combine with each other to produce O<sub>2</sub>, and they can combine with O<sub>2</sub> to produce O<sub>3</sub> (in a reverse of the above reaction). | ||
| − | == | + | == Tropospheric ozone == |
| − | + | === Formation === | |
| − | + | In the troposphere, ozone is produced from O<sub>2</sub> by many processes, including lightning strikes and combustion. Some types of electrical equipment generate significant levels of ozone. This is especially true of devices using [[high voltage]]s, such as [[television]] sets, [[laser printer]]s, and [[photocopier]]s. Electric motors using [[brush (electric)|brush]]es can generate ozone from repeated sparking inside the unit. Large motors, such as those used by elevators or hydraulic pumps, will generate more ozone than smaller motors. In addition, ozone is naturally produced by [[white blood cell]]s and the roots of [[marigold]]s as a means of destroying foreign bodies. | |
| − | [[ | + | |
| − | === | + | Much of the ozone in the troposphere is formed when nitrogen oxides (NOx), carbon monoxide (CO), and volatile organic compounds (VOCs; a mixture of hydrocarbons) react in the atmosphere in the presence of sunlight. NOx and VOCs are called ozone precursors. Motor vehicle exhaust, industrial emissions, and chemical solvents are the major anthropogenic sources of these chemicals. Although these precursors often originate in urban areas, winds can carry NOx hundreds of kilometers, causing ozone formation to occur in less populated regions as well. The atmospheric concentration of methane, a VOC, has increased tremendously over the last century, and it contributes to ozone formation on a global scale. Thus, various human activities have raised the concentration of ozone in the troposphere. In addition, about 10 percent of the ozone comes from the stratosphere (which lies just above the troposphere). |
| − | '' | + | |
| + | Hydrocarbons, nitrogen oxides, and ozone are the major components of smog that frequently occurs in urban and suburban areas. Recent satellite maps of [[nitrogen dioxide]] (NO<sub>2</sub>) clearly show the worldwide distribution of polluted regions associated with emissions from automobiles, factories, and power plants that burn fossil fuels. | ||
| + | |||
| + | === Health effects === | ||
| + | |||
| + | Relatively high concentrations of ozone at ground level can have the following health effects: | ||
| + | * Irritation of the [[respiratory system]], causing coughing, throat irritation, and/or an uncomfortable sensation in the chest. | ||
| + | * Reduced [[lung]] function, making it more difficult to breathe deeply and vigorously. Breathing may become more rapid and more shallow than normal, and a person's ability to engage in vigorous activities may be limited. | ||
| + | * Aggravation of [[asthma]]. When ozone levels are high, more people with asthma have attacks that require a doctor's attention or use of medication. One reason this happens is that ozone makes people more sensitive to [[allergen]]s, which in turn trigger asthma attacks. | ||
| + | * Increased susceptibility to [[Upper respiratory tract infection|respiratory infections]]. | ||
| + | * Inflammation and damage to the lining of the lungs. Within a few days, the damaged cells are shed and replaced, much like the skin peels off after a sunburn. Animal studies suggest that if this type of inflammation happens repeatedly over a long time period (months, years, a lifetime), lung tissue may become permanently scarred, resulting in permanent loss of lung function and a lower quality of life. | ||
| + | * Conversion of [[cholesterol]] in the bloodstream to plaque, which causes hardening and narrowing of arteries. | ||
| + | |||
| + | A statistical study of 95 large urban communities in the United States found significant association between ozone levels and premature death. The study estimated that a one-third reduction in urban ozone concentrations would save roughly 4,000 lives per year (Bell et. al, 2004). Air quality guidelines, such as those from the [[World Health Organization]] (WHO), are based on detailed studies of what levels can cause measurable health effects. | ||
| + | |||
| + | There is also evidence of significant reduction in agricultural yields due to increased ground-level ozone, which interferes with [[photosynthesis]] and stunts overall growth of some plant species [http://earthobservatory.nasa.gov/Newsroom/MediaAlerts/2003/2003073015111.html][http://www.arb.ca.gov/research/abstracts/94-345.htm]. | ||
| + | |||
| + | Although ozone was present at ground level before the industrial revolution, peak concentrations are currently far higher than pre-industrial levels [http://reports.eea.eu.int/TOP08-98/en/page004.html]. In addition, background concentrations well away from sources of pollution are substantially higher [http://www.grida.no/climate/ipcc_tar/wg1/142.htm]. | ||
| + | |||
| + | Ozone is a powerful [[oxidizing agent]] readily reacting with other chemical compounds to make many possibly [[toxic]] oxides. In addition, ozone reacts directly with some [[hydrocarbon]]s (of the type known as ''alkenes'') to produce compounds known as [[aldehyde]]s and [[ketone]]s. This process, called ''ozonolysis'', helps lower the amounts of hydrocarbons and ozone in the air, but the products of the ozonolysis are themselves key components of [[smog]]. | ||
| + | |||
| + | Another reaction of ozone, called ''photolysis'' by UV light, leads to production of the [[hydroxyl radical]] (OH), which plays a part in the removal of hydrocarbons from the air, but is again a step in the creation of components of smog such as [[peroxyacyl nitrates]], which are powerful eye irritants. Ultimately, ozone is one component of smog that is harmful in itself and contributes to both the production and removal of other air pollutants. | ||
| + | |||
| + | == Ozone layer == | ||
| − | |||
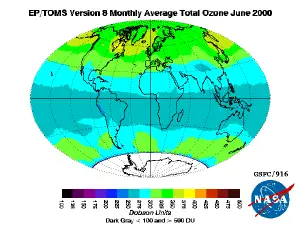
[[Image:IM_ozavg_ept_200006.png|thumb|Total ozone concentration in June 2000 as measured by EP-TOMS satellite instrument.]] | [[Image:IM_ozavg_ept_200006.png|thumb|Total ozone concentration in June 2000 as measured by EP-TOMS satellite instrument.]] | ||
| − | |||
| − | |||
| − | + | The '''ozone layer''' is the region of the [[Earth's atmosphere#stratosphere|Earth's stratosphere]] that contains relatively high concentrations of ozone. These concentrations are greatest at altitudes between about 15 and 40 km, where they range from about 2 to 8 parts per million (ppm)—much higher than the ozone concentrations in the [[Earth's atmosphere#troposphere|troposphere]], but still small compared to the atmosphere's main components. | |
| + | |||
| + | The "thickness" of the ozone layer—that is, the total amount of ozone in a column overhead—varies by a large factor worldwide, generally being smaller near the equator and larger as one moves toward the poles. It also varies with season, being in general thicker during the spring and thinner during the autumn. The reasons for this latitude and seasonal dependence are complicated, involving atmospheric circulation patterns as well as solar intensity. | ||
| − | + | The ozone layer was discovered in 1913 by French physicists Charles Fabry and Henri Buisson. Its properties were explored in detail by British meteorologist [[G. M. B. Dobson]], who developed a simple spectrophotometer that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations that continues to operate today. | |
| − | + | The standard way to express total ozone amounts in the atmosphere is in terms of the "Dobson unit," which measures the total amount of ozone in a column overhead. When used in industry, ozone is measured in parts per million and percent by mass or weight. | |
| − | + | === Origin of ozone layer === | |
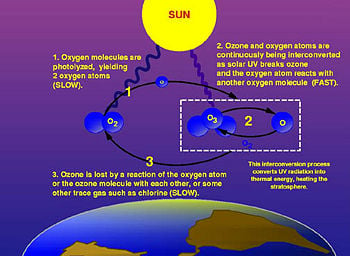
| + | [[Image:Ozone cycle.jpg|thumb|350px|The ozone-oxygen cycle in the ozone layer.]] | ||
| − | + | The photochemical mechanisms that give rise to the ozone layer were worked out by the British physicist Sidney Chapman in 1930. When [[ultraviolet]] (UV) light strikes dioxygen [[molecule]]s (O<sub>2</sub>), they split up into individual oxygen atoms (atomic oxygen). The atomic oxygen then combines with unbroken O<sub>2</sub> to create ozone, O<sub>3</sub>. Given that the ozone molecule is unstable (although relatively long-lived in the stratosphere), when it is hit by UV light, it splits into a molecule of O<sub>2</sub> and an atom of oxygen. These processes, which occur repetitively, are together called the ''ozone-oxygen cycle'' and create an ozone layer in the stratosphere. | |
| − | == | + | === Ultraviolet light and ozone === |
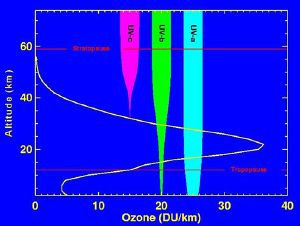
| − | + | [[Image:Ozone altitude UV graph.jpg|left|thumb|Levels of ozone at various altitudes and blocking of ultraviolet radiation.]] | |
| − | |||
| − | |||
| − | |||
| − | + | Although the concentration of ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful UV radiation emitted from the Sun. UV radiation is divided into three categories, based on its wavelength: UV-A, UV-B, and UV-C. UV-C, which would be extremely harmful to humans, is entirely screened out by ozone at around 35 km altitude. | |
| + | |||
| + | UV-B radiation is the main cause of [[sunburn]]; excessive exposure can also cause genetic damage, resulting in problems such as skin cancer. The ozone layer is very effective at screening out most of the UV-B; for UV-B radiation with a wavelength of 290 nm, the intensity at the Earth's surface is 350 million times weaker than at the top of the atmosphere. Nevertheless, some UV-B reaches the surface. Most UV-A reaches the surface; this radiation is significantly less harmful, although it can potentially cause genetic damage. | ||
| + | |||
| + | Depletion of the ozone layer would allow more of the UV radiation, and particularly the more harmful wavelengths, to reach the surface, causing increased genetic damage to living things. | ||
| + | |||
| + | === DNA sensitivity to UV === | ||
| + | |||
| + | There is much greater [[probability]] of [[DNA]] damage by UV radiation at various wavelengths. Fortunately, where DNA is easily damaged, such as by wavelengths shorter than 290 nm, ozone strongly absorbs UV. At the longer wavelengths where ozone absorbs weakly, DNA damage is less likely. If there was a 10 percent decrease in ozone, the amount of DNA damaging UV increases, in this case, by about 22 percent. Considering that DNA damage can lead to maladies like skin cancer, it is clear that this absorption of the Sun's UV radiation by ozone is critical for our well-being. | ||
| + | |||
| + | === Distribution of ozone in the stratosphere === | ||
| + | |||
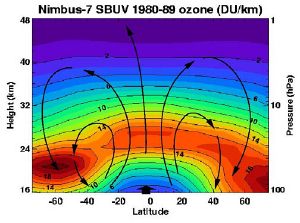
| + | [[Image:Nimbus ozone Brewer-Dobson circulation.jpg|thumb|Brewer-Dobson circulation in the ozone layer.]] | ||
| + | |||
| + | Most of the stratospheric ozone is created over the tropics, but then stratospheric wind patterns, known as the "Brewer-Dobson circulation," transport the ozone poleward and downward to the lower stratosphere of the high latitudes. Consequently, most of the ozone is found in the mid-to-high latitudes of the northern and southern hemispheres; the highest levels are found in the spring, not summer, and the lowest in the fall, not winter. Moreover, the ozone layer is higher in altitude in the tropics, and lower in altitude beyond the tropics, especially in the polar regions. | ||
| + | |||
| + | Over the continental [[United States]] (25° N to 49° N), stratospheric ozone amounts are highest in the spring (April and May). These amounts fall over the course of the summer to their lowest levels in October, then rise again over the course of the winter. Again, wind transport of ozone is principally responsible for the seasonal changes of these higher latitude ozone patterns. | ||
| + | |||
| + | The total column amount of ozone generally increases as we move from the tropics to higher latitudes in both hemispheres. However, the overall column amounts are greater in the northern hemisphere high latitudes than in the southern hemisphere high latitudes. The highest amounts of column ozone anywhere in the world are found over the Arctic region during the northern spring period of March and April. The amounts then decrease over the course of the northern summer. Meanwhile, the lowest amounts of column ozone anywhere in the world are found over the Antarctic in the southern spring period of September and October (see "ozone hole" mentioned below). | ||
| + | |||
| + | ==Ozone depletion== | ||
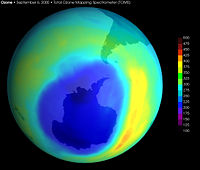
| + | [[Image:Largest ever Ozone hole sept2000 with scale.jpg|thumb|right|200px|Image of the largest Antarctic ozone hole observed, recorded in September 2000. Data taken by the Total Ozone Mapping Spectrometer (TOMS) instrument aboard NASA's Earth Probe satellite.]] | ||
| + | |||
| + | The term '''ozone depletion''' is used to describe two distinct but related observations: (a) a slow, steady decline, of about 3 percent per decade, in the total amount of ozone in the Earth's stratosphere during the past 20 years; and (b) a much larger, but seasonal, decrease in stratospheric ozone over the Earth's polar regions during the same period. The latter phenomenon is commonly referred to as the "ozone hole." | ||
| + | |||
| + | The detailed mechanism of formation of polar ozone holes is different from that for the mid-latitude thinning, but both trends are believed to be caused by destruction of ozone by a number of [[free radical]] catalysts—particularly hydroxyl (OH), nitric oxide (NO), atomic [[chlorine]] (Cl), and atomic [[bromine]] (Br). | ||
| + | |||
| + | At present, most of the OH and NO in the stratosphere are of natural origin, while the concentrations of Cl and Br atoms (classified as "halogen" atoms) have risen through human activity. It appears that the halogen atoms in the stratosphere are formed mainly by the UV-catalyzed breakdown of chlorofluorocarbon (CFC) compounds, commonly called Freons, and bromofluorocarbon compounds, known as Halons, which are transported into the stratosphere after being emitted at the surface. | ||
| + | |||
| + | The free Cl or Br atoms can catalyze the conversion of ozone (O<sub>3</sub>) to oxygen molecules (O<sub>2</sub>). The chemical reactions catalyzed by Cl atoms can be written as follows: | ||
| + | :Cl + O<sub>3</sub> —> ClO + O<sub>2</sub> | ||
| + | :ClO + O —> Cl + O<sub>2</sub> | ||
| + | The overall conversion reaction is: | ||
| + | :O<sub>3</sub> + O —> O<sub>2</sub> + O<sub>2</sub> | ||
| + | For this mechanism to operate, there must be a source of O atoms, and these are produced by the breakup of O<sub>3</sub> molecules by UV light. | ||
| + | |||
| + | A single chlorine atom could keep on destroying ozone for up to two years (the time scale for transport back down to the troposphere), were it not for reactions that remove Cl from this cycle by forming compounds such as [[hydrochloric acid]]. On a per-atom basis, bromine is even more efficient than chlorine at destroying ozone, but there is much less bromine in the atmosphere. | ||
| + | |||
| + | Given that the ozone layer prevents harmful UVC and UVB wavelengths of light from passing through the [[Earth's atmosphere]], observed and projected decreases in ozone have generated worldwide concern. This concern has led to adoption of the Montreal Protocol, which bans the production of CFCs and halons, as well as related ozone-depleting chemicals such as [[carbon tetrachloride]] and 1,1,1-trichloroethane (also known as methyl chloroform). It is suspected that increased UV exposure due to ozone depletion may have a variety of biological consequences, including increases in [[skin cancer]], damage to plants, and reduction of [[plankton]] populations in the oceans. | ||
| + | |||
| + | ==Industrial and laboratory production== | ||
| + | |||
| + | Industrially, ozone is produced by subjecting oxygen in the air to either (a) short-[[wavelength]] UV radiation using a [[mercury vapor|mercury vapor lamp]], or (b) a high-[[voltage]] electric field in a process called ''cold discharge'' or ''corona discharge''. The cold discharge apparatus consists of two metal plates separated by an air gap and an electrical insulator (such as borosilicate glass or mica). When a high-voltage alternating current is applied to the plates, ozone is formed in the air gap, as O<sub>2</sub> molecules dissociate and recombine into O<sub>3</sub>. | ||
| + | |||
| + | In the laboratory, ozone can be produced by [[electrolysis]] (electrical breakup) of acidified water. A pencil graphite rod [[cathode]] and a [[platinum]] wire [[anode]] are dipped in a solution containing [[sulfuric acid]] (at a concentration of 3 Molar), and the electrodes are connected to a 9-volt [[battery]] to generate an electrical current. In the overall reaction, three equivalents of water are converted into one equivalent of ozone and one equivalent of [[hydrogen]]. A competing reaction is the formation of [[oxygen]]. (See Jorge G. Ibanez et al., 2005, in [[#References|References]] below). | ||
==Reactions== | ==Reactions== | ||
| − | Ozone will oxidize metals (except [[gold]], [[platinum]], and [[iridium]]) to [[oxide|oxides]] of the metals in their highest [[oxidation state]]: | + | |
| + | Ozone is a reagent for many reactions in the laboratory and industry. Some of these are listed here. | ||
| + | |||
| + | Ozone will oxidize metals (except [[gold]], [[platinum]], and [[iridium]]) to [[oxide|oxides]] of the metals in their highest [[oxidation state]]. For example, cobalt ions are oxidized from Co<sup>2+</sup> to Co<sup>3+</sup> as follows: | ||
:2 Co<sup>2+</sup> + 2 H<sup>+</sup> + O<sub>3</sub> → 2 Co<sup>3+</sup> + H<sub>2</sub>O + O<sub>2</sub> | :2 Co<sup>2+</sup> + 2 H<sup>+</sup> + O<sub>3</sub> → 2 Co<sup>3+</sup> + H<sub>2</sub>O + O<sub>2</sub> | ||
| − | Ozone oxidizes oxides to [[peroxide|peroxides]] | + | |
| + | Ozone oxidizes oxides to [[peroxide|peroxides]], or to oxides of higher oxidation number. For example, sulfur dioxide (SO<sub>2</sub>) is converted to sulfur trioxide (SO<sub>3</sub>), and nitric oxide (NO) is converted to nitrogen dioxide (NO<sub>2</sub>), as follows: | ||
:SO<sub>2</sub> + O<sub>3</sub> → SO<sub>3</sub> + O<sub>2</sub> | :SO<sub>2</sub> + O<sub>3</sub> → SO<sub>3</sub> + O<sub>2</sub> | ||
:NO + O<sub>3</sub> → NO<sub>2</sub> + O<sub>2</sub> | :NO + O<sub>3</sub> → NO<sub>2</sub> + O<sub>2</sub> | ||
| − | The above reaction is accompanied by [[chemiluminescence]]. The NO<sub>2</sub> can be further oxidized: | + | The above reaction is accompanied by [[chemiluminescence]]. The NO<sub>2</sub> can be further oxidized to NO<sub>3</sub>: |
:NO<sub>2</sub> + O<sub>3</sub> → NO<sub>3</sub> + O<sub>2</sub> | :NO<sub>2</sub> + O<sub>3</sub> → NO<sub>3</sub> + O<sub>2</sub> | ||
The NO<sub>3</sub> formed can react with NO<sub>2</sub> to form N<sub>2</sub>O<sub>5</sub>: | The NO<sub>3</sub> formed can react with NO<sub>2</sub> to form N<sub>2</sub>O<sub>5</sub>: | ||
:NO<sub>2</sub> + NO<sub>3</sub> → N<sub>2</sub>O<sub>5</sub> | :NO<sub>2</sub> + NO<sub>3</sub> → N<sub>2</sub>O<sub>5</sub> | ||
| + | |||
Ozone reacts with [[carbon]] to form [[carbon dioxide]], even at room temperature: | Ozone reacts with [[carbon]] to form [[carbon dioxide]], even at room temperature: | ||
:C + 2 O<sub>3</sub> → CO<sub>2</sub> + 2 O<sub>2</sub> | :C + 2 O<sub>3</sub> → CO<sub>2</sub> + 2 O<sub>2</sub> | ||
| − | Ozone does not react with ammonium [[salt|salts]] but it reacts with [[ammonia]] to form | + | |
| + | Ozone does not react with ammonium [[salt|salts]] but it reacts with [[ammonia]] (NH<sub>3</sub>) to form ammonium nitrate (NH<sub>4</sub>NO<sub>3</sub>): | ||
:NH<sub>3</sub> + 4 O<sub>3</sub> → NH<sub>4</sub>NO<sub>3</sub> + 4 O<sub>3</sub> + H<sub>2</sub>0 | :NH<sub>3</sub> + 4 O<sub>3</sub> → NH<sub>4</sub>NO<sub>3</sub> + 4 O<sub>3</sub> + H<sub>2</sub>0 | ||
| − | Ozone reacts with [[sulfide|sulfides]] to make [[sulfate|sulfates]]: | + | |
| + | Ozone reacts with [[sulfide|sulfides]] to make [[sulfate|sulfates]]. For instance, [[lead]] sulfide (PbS) is converted to lead sulfate (PbSO<sub>4</sub>): | ||
:PbS + 4 O<sub>3</sub> → PbSO<sub>4</sub> + 4 O<sub>2</sub> | :PbS + 4 O<sub>3</sub> → PbSO<sub>4</sub> + 4 O<sub>2</sub> | ||
| − | [[ | + | |
| + | Ozone can react with [[sulfur]] (S) or [[sulfur dioxide]] (SO<sub>2</sub>) to produce [[sulfuric acid]] (H<sub>2</sub>SO<sub>4</sub>): | ||
:S + H<sub>2</sub>O + O<sub>3</sub> → H<sub>2</sub>SO<sub>4</sub> | :S + H<sub>2</sub>O + O<sub>3</sub> → H<sub>2</sub>SO<sub>4</sub> | ||
:3 SO<sub>2</sub> + 3 H<sub>2</sub>O + O<sub>3</sub> → 3 H<sub>2</sub>SO<sub>4</sub> | :3 SO<sub>2</sub> + 3 H<sub>2</sub>O + O<sub>3</sub> → 3 H<sub>2</sub>SO<sub>4</sub> | ||
| − | All three [[atom|atoms]] of ozone may also react, as in the reaction with [[tin]](II) chloride and [[hydrochloric acid]]: | + | |
| + | All three [[atom|atoms]] of ozone may also react, as in the reaction with [[tin]](II) chloride (SnCl<sub>2</sub>) and [[hydrochloric acid]] (HCl): | ||
:3 SnCl<sub>2</sub> + 6 HCl + O<sub>3</sub> → 3 SnCl<sub>4</sub> + 3 H<sub>2</sub>O | :3 SnCl<sub>2</sub> + 6 HCl + O<sub>3</sub> → 3 SnCl<sub>4</sub> + 3 H<sub>2</sub>O | ||
| − | + | ||
| − | + | Ozone can be used for [[combustion]] reactions, and the burning of gases in ozone produces higher temperatures than burning them in dioxygen (O<sub>2</sub>). Following is a reaction for the combustion of carbon subnitride (C<sub>4</sub>N<sub>2</sub>): | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | Ozone can be used for [[combustion]] reactions and | ||
:3 C<sub>4</sub>N<sub>2</sub> + 4 O<sub>3</sub> → 12 CO + 3 N<sub>2</sub> | :3 C<sub>4</sub>N<sub>2</sub> + 4 O<sub>3</sub> → 12 CO + 3 N<sub>2</sub> | ||
| − | Ozone can react at cryogenic temperatures. At 77 K (- | + | |
| + | Ozone can react at cryogenic (very low) temperatures. At 77 K (-196°C), atomic [[hydrogen]] reacts with liquid ozone to form a hydrogen [[superoxide]] [[radical (chemistry)|radical]] (HO<sub>2</sub>), which converts to the dimer H<sub>2</sub>O<sub>4</sub> (M. Horvath et al., 1985, pp. 44-49, referenced below): | ||
:H + O<sub>3</sub> → HO<sub>2</sub> + O | :H + O<sub>3</sub> → HO<sub>2</sub> + O | ||
:2 HO<sub>2</sub> → H<sub>2</sub>O<sub>4</sub> | :2 HO<sub>2</sub> → H<sub>2</sub>O<sub>4</sub> | ||
| − | + | ||
| + | It is also possible to form compounds called ozonides, which contain the ozonide anion (O<sub>3</sub><sup>-</sup>). These compounds are explosive and must be stored at cryogenic temperatures. Ozonides for all the [[alkali metal]]s are known. KO<sub>3</sub>, RbO<sub>3</sub>, and CsO<sub>3</sub> can be prepared from their respective superoxides. For example, KO<sub>3</sub> can be formed from KO<sub>2</sub>. | ||
:KO<sub>2</sub> + O<sub>3</sub> → KO<sub>3</sub> + O<sub>2</sub> | :KO<sub>2</sub> + O<sub>3</sub> → KO<sub>3</sub> + O<sub>2</sub> | ||
| − | + | NaO<sub>3</sub> and LiO<sub>3</sub> must be prepared by action of CsO<sub>3</sub> in liquid ammonia (NH<sub>3</sub>) on an ion exchange resin containing Na<sup>+</sup> or Li<sup>+</sup> ions (Housecroft & Sharpe, 2005, p. 265, referenced below): | |
| − | |||
| − | |||
| − | NaO<sub>3</sub> and LiO<sub>3</sub> must be prepared by action of CsO<sub>3</sub> in liquid NH<sub>3</sub> on an ion exchange resin containing Na<sup>+</sup> or Li<sup>+</sup> ions | ||
:CsO<sub>3</sub> + Na<sup>+</sup> → Cs<sup>+</sup> + NaO<sub>3</sub> | :CsO<sub>3</sub> + Na<sup>+</sup> → Cs<sup>+</sup> + NaO<sub>3</sub> | ||
| − | + | ||
| − | + | Ozone can be used to remove [[manganese]] (Mn<sup>2+</sup>) ions from [[water]], by forming a [[precipitate]] of MnO(OH)<sub>2</sub>, which can be filtered: | |
| − | + | :2 Mn<sup>2+</sup> + 2 O<sub>3</sub> + 4 H<sub>2</sub>O → 2 MnO(OH)<sub>2</sub> (s) + 2 O<sub>2</sub> + 4 H<sup>+</sup> | |
| − | :2 Mn<sup>2+</sup> + 2 O<sub>3</sub> + 4 H<sub>2</sub>O → 2 MnO(OH)<sub>2 | + | |
| − | Ozone will also turn [[cyanide|cyanides]] to the | + | Ozone will also turn [[cyanide|cyanides]] (CN<sup>-</sup>) to the 1,000-times less toxic [[cyanate|cyanates]] (CNO<sup>-</sup>): |
:CN<sup>-</sup> + O<sub>3</sub> → CNO<sup>-</sup> + O<sub>2</sub> | :CN<sup>-</sup> + O<sub>3</sub> → CNO<sup>-</sup> + O<sub>2</sub> | ||
| − | Finally, ozone will | + | |
| + | Finally, ozone will completely decompose [[urea]] ((NH<sub>2</sub>)<sub>2</sub>CO) (M. Horvath et al., 1985, pp. 259, 269-270, referenced below): | ||
:(NH<sub>2</sub>)<sub>2</sub>CO + O<sub>3</sub> → N<sub>2</sub> + CO<sub>2</sub> + 2 H<sub>2</sub>O | :(NH<sub>2</sub>)<sub>2</sub>CO + O<sub>3</sub> → N<sub>2</sub> + CO<sub>2</sub> + 2 H<sub>2</sub>O | ||
| − | == | + | ==Uses of ozone== |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | == | + | ===Municipal water treatment=== |
| − | Ozone | + | Ozone can be used for [[Bleach (chemical)|bleach]]ing materials and killing bacteria. Many municipal drinking water systems kill bacteria with ozone instead of the more common [[chlorine]]. Unlike chlorine, ozone does not form organochlorine compounds (which can be harmful), and ozone does not remain in the water after treatment. Some systems introduce a small amount of chlorine to prevent bacterial growth in the pipes, or may use chlorine intermittently, based on the results of periodic testing. Ozone is also popularly used in spas or hot tubs instead of chlorine or [[bromine]] to keep the water free of bacteria. |
| − | + | In places where electrical power is abundant, ozone is a cost-effective method of treating water, as it is produced on demand and does not require transportation and storage of hazardous chemicals. Once it has decayed, it leaves no taste or odor in drinking water. | |
| − | [[ | + | ===Industrial uses=== |
| + | Industrially, ozone or ozonated water is used for a variety purposes, such as: | ||
| + | * to disinfect water before it is bottled; | ||
| + | * to kill bacteria on food-contact surfaces; | ||
| + | * to scrub yeast and mold spores from the air in food-processing plants; | ||
| + | * to wash fresh fruits and vegetables to kill yeast, mold, and bacteria; | ||
| + | * to chemically attack contaminants in water ([[iron]], [[arsenic]], [[hydrogen sulfide]], [[nitrite]]s, and complex organics lumped together as "color"); | ||
| + | * to provide an aid to [[flocculation]] (a process of agglomeration of molecules, which aids in filtration—a process by which iron and arsenic are removed); | ||
| + | * to clean and bleach fabrics (the latter process is patented); | ||
| + | * to assist in processing plastics to allow the adhesion of inks; and | ||
| + | * to age rubber samples when determining the useful life of a batch of rubber. | ||
| − | + | ===Medical uses=== | |
| + | Ozone has a number of uses in the medical arena. For instance, many hospitals around the world use large ozone generators to decontaminate operating rooms between surgeries. The rooms are cleaned, then sealed airtight and filled with ozone, which effectively kills or neutralizes all remaining bacteria. | ||
| − | + | Ozone can be used to affect the body's [[antioxidant]]-prooxidant balance, because the body usually reacts to its presence by producing antioxidant enzymes. Ozone therapy has blossomed into a thriving field of [[alternative medicine]], and there are a host of claimed applications above and beyond what has actually been verified by studies. | |
| − | + | The U.S. [[Food and Drug Administration]] (FDA) has not approved the use of ozone therapy on humans. Nonetheless, at least 12 states (AK, AZ, CO, GA, MN, NY, NC, OH, OK, OR, SC and WA) have passed legislation to ensure that alternative therapies are available to consumers. Physicians in those states can legally use ozone as an alternative treatment in their practice, without fear of prosecution. In addition, medical ozone therapy is recognized in Bulgaria, Cuba, Czech Republic, France, Germany, Israel, Italy, Mexico, Romania, and Russia. | |
| − | |||
| − | + | At least one death has been attributed to the application of ozone through [[Wiktionary:insufflation|insufflation]] in the United States. Nonetheless, "air cleaners" that produce "activated oxygen" (that is, ozone) are often sold. | |
==See also== | ==See also== | ||
| − | * [ | + | * [http://www.ccme.ca/assets/pdf/pmozone_standard_e.pdf Canada-wide standards for particulate matter (PM2.5) and ozone] (pdf) |
| − | * [[ | + | * [[National Ambient Air Quality Standards]] (USA) |
| − | * [[ | + | * [[Photochemical smog]] |
==Further reading== | ==Further reading== | ||
| − | * | + | |
| − | + | * Greenwood, N. N. and A. Earnshaw. 1997. ''Chemistry of the Elements'' (2nd Ed.). Oxford: Butterworth-Heinemann. ISBN 0750633654 | |
==References== | ==References== | ||
| − | + | * Bell, Michelle L., Aidan McDermott, Scott L. Zeger, Jonathan M. Samet, Francesca Dominici (2004). "Ozone and Short-term Mortality in 95 US Urban Communities, 1987-2000." ''Journal of the American Medical Association'' 292: 2372-2378. | |
| − | + | * Ibanez, Jorge G., Rodrigo Mayen-Mondragon, and M. T. Moran-Moran: "Laboratory Experiments on the Electrochemical Remediation of the Environment. Part 7: Microscale Production of Ozone," ''J. Chem. Ed''. 82:10 (October 2005): 1546 [http://jchemed.chem.wisc.edu/Journal/Issues/2005/Oct/abs1546.html Abstract]. | |
| + | * Horvath, M., L. Bilitzky, & J. Huttner. 1985. "Ozone." | ||
| + | * Housecroft & Sharpe. 2005. "Inorganic Chemistry." | ||
| + | * Seinfeld, John H., and Spyros N. Pandis. 1998. Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 0471178160 | ||
| + | * Wayne, Richard P. 2000. ''Chemistry of Atmospheres'' (3rd Ed.). Oxford University Press. ISBN 0-19-850375-X | ||
==External links== | ==External links== | ||
| − | + | All links retrieved November 18, 2022. | |
| − | + | ||
| − | |||
*[http://www.cdc.gov/niosh/npg/npgd0476.html NIOSH Pocket Guide to Chemical Hazards] | *[http://www.cdc.gov/niosh/npg/npgd0476.html NIOSH Pocket Guide to Chemical Hazards] | ||
| − | + | *[http://www.greenfacts.org/air-pollution/ozone-o3/index.htm Air Pollution Ozone] | |
| − | + | *[http://airnow.gov/ U.S. Environmental Protection Agency Live Ozone Map] | |
| − | *[http://www.greenfacts.org/air-pollution/ozone-o3/index.htm | ||
| − | *[http:// | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | [[ | + | [[Category:Physical sciences]] |
| − | [[ | + | [[Category:Chemistry]] |
| − | + | {{credits|Ozone|52080748|Ozone_layer|52035301|Ozone_depletion|52274160|Tropospheric_ozone|50878181}} | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
Latest revision as of 06:05, 18 November 2022
| Ozone | |
|---|---|
| |
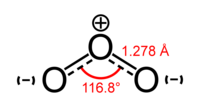
| General | |
| Systematic name | Trioxygen |
| Molecular formula | O3 |
| Molar mass | 47.998 g/mol |
| Appearance | bluish colored gas |
| CAS number | [10028-15-6] |
| Properties | |
| Density and phase | 2.144 g/l (0 °C), gas |
| Solubility in water | 0.105 g/100 ml (0 °C) |
| Melting point | 75.95 K, −197.2 °C |
| Boiling point | 161.25 K, −111.9 °C |
| Thermodynamic data | |
| Standard enthalpy of formation ΔfH°solid |
+142.3 kJ/mol |
| Standard molar entropy S°solid |
237.7 J.K−1.mol−1 |
| Hazards | |
| EU classification | not listed |
| NFPA 704 | |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Regulatory data | Flash point, RTECS number, etc. |
| Except where noted otherwise, data are given for materials in their standard state (at 25° C, 100 kPa pressure) | |
Ozone (molecular formula O3) is a minor constituent of the Earth's atmosphere, but its effects are highly significant. It is chemically very reactive and is involved in reactions that drive many of the chemical changes that occur in the atmosphere by day and by night.
About 90 percent of the ozone in our atmosphere is contained in the stratosphere (part of the upper atmosphere), and about 10 percent is contained in the troposphere (lower atmosphere). Ground-level ozone is an air pollutant with harmful effects on our respiratory system. On the other hand, ozone in the upper atmosphere protects living organisms by preventing damaging ultraviolet light from reaching the Earth's surface.
Discovery and notable characteristics
Ozone was discovered in 1840 by Christian Friedrich Schönbein, who named it after the Greek word for smell (ozein), associating it with the peculiar odor in the air after lightning storms. [1]. The odor from a lightning strike, however, is from electrons freed during rapid chemical changes, not from the ozone itself [2].
Each molecule of ozone consists of three oxygen atoms, and its molecular formula is therefore written as O3. As such, it is an allotrope of oxygen (dioxygen, O2), which is a much more stable and abundant gas.
At standard temperature and pressure (0°C and 100 kilopascals pressure), ozone is a pale blue gas. It forms a dark blue liquid below −112° C and a dark blue solid below −193° C. It is a powerful oxidizing agent (see Reactions below).
Ozone is unstable, and when it breaks down, it gives rise to ordinary oxygen (O2) and free radicals of atomic oxygen (O). The reaction is as follows.
- O3 → O2 + O
The free radicals are highly reactive and damage or destroy most organic molecules. They can also combine with each other to produce O2, and they can combine with O2 to produce O3 (in a reverse of the above reaction).
Tropospheric ozone
Formation
In the troposphere, ozone is produced from O2 by many processes, including lightning strikes and combustion. Some types of electrical equipment generate significant levels of ozone. This is especially true of devices using high voltages, such as television sets, laser printers, and photocopiers. Electric motors using brushes can generate ozone from repeated sparking inside the unit. Large motors, such as those used by elevators or hydraulic pumps, will generate more ozone than smaller motors. In addition, ozone is naturally produced by white blood cells and the roots of marigolds as a means of destroying foreign bodies.
Much of the ozone in the troposphere is formed when nitrogen oxides (NOx), carbon monoxide (CO), and volatile organic compounds (VOCs; a mixture of hydrocarbons) react in the atmosphere in the presence of sunlight. NOx and VOCs are called ozone precursors. Motor vehicle exhaust, industrial emissions, and chemical solvents are the major anthropogenic sources of these chemicals. Although these precursors often originate in urban areas, winds can carry NOx hundreds of kilometers, causing ozone formation to occur in less populated regions as well. The atmospheric concentration of methane, a VOC, has increased tremendously over the last century, and it contributes to ozone formation on a global scale. Thus, various human activities have raised the concentration of ozone in the troposphere. In addition, about 10 percent of the ozone comes from the stratosphere (which lies just above the troposphere).
Hydrocarbons, nitrogen oxides, and ozone are the major components of smog that frequently occurs in urban and suburban areas. Recent satellite maps of nitrogen dioxide (NO2) clearly show the worldwide distribution of polluted regions associated with emissions from automobiles, factories, and power plants that burn fossil fuels.
Health effects
Relatively high concentrations of ozone at ground level can have the following health effects:
- Irritation of the respiratory system, causing coughing, throat irritation, and/or an uncomfortable sensation in the chest.
- Reduced lung function, making it more difficult to breathe deeply and vigorously. Breathing may become more rapid and more shallow than normal, and a person's ability to engage in vigorous activities may be limited.
- Aggravation of asthma. When ozone levels are high, more people with asthma have attacks that require a doctor's attention or use of medication. One reason this happens is that ozone makes people more sensitive to allergens, which in turn trigger asthma attacks.
- Increased susceptibility to respiratory infections.
- Inflammation and damage to the lining of the lungs. Within a few days, the damaged cells are shed and replaced, much like the skin peels off after a sunburn. Animal studies suggest that if this type of inflammation happens repeatedly over a long time period (months, years, a lifetime), lung tissue may become permanently scarred, resulting in permanent loss of lung function and a lower quality of life.
- Conversion of cholesterol in the bloodstream to plaque, which causes hardening and narrowing of arteries.
A statistical study of 95 large urban communities in the United States found significant association between ozone levels and premature death. The study estimated that a one-third reduction in urban ozone concentrations would save roughly 4,000 lives per year (Bell et. al, 2004). Air quality guidelines, such as those from the World Health Organization (WHO), are based on detailed studies of what levels can cause measurable health effects.
There is also evidence of significant reduction in agricultural yields due to increased ground-level ozone, which interferes with photosynthesis and stunts overall growth of some plant species [3][4].
Although ozone was present at ground level before the industrial revolution, peak concentrations are currently far higher than pre-industrial levels [5]. In addition, background concentrations well away from sources of pollution are substantially higher [6].
Ozone is a powerful oxidizing agent readily reacting with other chemical compounds to make many possibly toxic oxides. In addition, ozone reacts directly with some hydrocarbons (of the type known as alkenes) to produce compounds known as aldehydes and ketones. This process, called ozonolysis, helps lower the amounts of hydrocarbons and ozone in the air, but the products of the ozonolysis are themselves key components of smog.
Another reaction of ozone, called photolysis by UV light, leads to production of the hydroxyl radical (OH), which plays a part in the removal of hydrocarbons from the air, but is again a step in the creation of components of smog such as peroxyacyl nitrates, which are powerful eye irritants. Ultimately, ozone is one component of smog that is harmful in itself and contributes to both the production and removal of other air pollutants.
Ozone layer
The ozone layer is the region of the Earth's stratosphere that contains relatively high concentrations of ozone. These concentrations are greatest at altitudes between about 15 and 40 km, where they range from about 2 to 8 parts per million (ppm)—much higher than the ozone concentrations in the troposphere, but still small compared to the atmosphere's main components.
The "thickness" of the ozone layer—that is, the total amount of ozone in a column overhead—varies by a large factor worldwide, generally being smaller near the equator and larger as one moves toward the poles. It also varies with season, being in general thicker during the spring and thinner during the autumn. The reasons for this latitude and seasonal dependence are complicated, involving atmospheric circulation patterns as well as solar intensity.
The ozone layer was discovered in 1913 by French physicists Charles Fabry and Henri Buisson. Its properties were explored in detail by British meteorologist G. M. B. Dobson, who developed a simple spectrophotometer that could be used to measure stratospheric ozone from the ground. Between 1928 and 1958, Dobson established a worldwide network of ozone monitoring stations that continues to operate today.
The standard way to express total ozone amounts in the atmosphere is in terms of the "Dobson unit," which measures the total amount of ozone in a column overhead. When used in industry, ozone is measured in parts per million and percent by mass or weight.
Origin of ozone layer
The photochemical mechanisms that give rise to the ozone layer were worked out by the British physicist Sidney Chapman in 1930. When ultraviolet (UV) light strikes dioxygen molecules (O2), they split up into individual oxygen atoms (atomic oxygen). The atomic oxygen then combines with unbroken O2 to create ozone, O3. Given that the ozone molecule is unstable (although relatively long-lived in the stratosphere), when it is hit by UV light, it splits into a molecule of O2 and an atom of oxygen. These processes, which occur repetitively, are together called the ozone-oxygen cycle and create an ozone layer in the stratosphere.
Ultraviolet light and ozone
Although the concentration of ozone in the ozone layer is very small, it is vitally important to life because it absorbs biologically harmful UV radiation emitted from the Sun. UV radiation is divided into three categories, based on its wavelength: UV-A, UV-B, and UV-C. UV-C, which would be extremely harmful to humans, is entirely screened out by ozone at around 35 km altitude.
UV-B radiation is the main cause of sunburn; excessive exposure can also cause genetic damage, resulting in problems such as skin cancer. The ozone layer is very effective at screening out most of the UV-B; for UV-B radiation with a wavelength of 290 nm, the intensity at the Earth's surface is 350 million times weaker than at the top of the atmosphere. Nevertheless, some UV-B reaches the surface. Most UV-A reaches the surface; this radiation is significantly less harmful, although it can potentially cause genetic damage.
Depletion of the ozone layer would allow more of the UV radiation, and particularly the more harmful wavelengths, to reach the surface, causing increased genetic damage to living things.
DNA sensitivity to UV
There is much greater probability of DNA damage by UV radiation at various wavelengths. Fortunately, where DNA is easily damaged, such as by wavelengths shorter than 290 nm, ozone strongly absorbs UV. At the longer wavelengths where ozone absorbs weakly, DNA damage is less likely. If there was a 10 percent decrease in ozone, the amount of DNA damaging UV increases, in this case, by about 22 percent. Considering that DNA damage can lead to maladies like skin cancer, it is clear that this absorption of the Sun's UV radiation by ozone is critical for our well-being.
Distribution of ozone in the stratosphere
Most of the stratospheric ozone is created over the tropics, but then stratospheric wind patterns, known as the "Brewer-Dobson circulation," transport the ozone poleward and downward to the lower stratosphere of the high latitudes. Consequently, most of the ozone is found in the mid-to-high latitudes of the northern and southern hemispheres; the highest levels are found in the spring, not summer, and the lowest in the fall, not winter. Moreover, the ozone layer is higher in altitude in the tropics, and lower in altitude beyond the tropics, especially in the polar regions.
Over the continental United States (25° N to 49° N), stratospheric ozone amounts are highest in the spring (April and May). These amounts fall over the course of the summer to their lowest levels in October, then rise again over the course of the winter. Again, wind transport of ozone is principally responsible for the seasonal changes of these higher latitude ozone patterns.
The total column amount of ozone generally increases as we move from the tropics to higher latitudes in both hemispheres. However, the overall column amounts are greater in the northern hemisphere high latitudes than in the southern hemisphere high latitudes. The highest amounts of column ozone anywhere in the world are found over the Arctic region during the northern spring period of March and April. The amounts then decrease over the course of the northern summer. Meanwhile, the lowest amounts of column ozone anywhere in the world are found over the Antarctic in the southern spring period of September and October (see "ozone hole" mentioned below).
Ozone depletion
The term ozone depletion is used to describe two distinct but related observations: (a) a slow, steady decline, of about 3 percent per decade, in the total amount of ozone in the Earth's stratosphere during the past 20 years; and (b) a much larger, but seasonal, decrease in stratospheric ozone over the Earth's polar regions during the same period. The latter phenomenon is commonly referred to as the "ozone hole."
The detailed mechanism of formation of polar ozone holes is different from that for the mid-latitude thinning, but both trends are believed to be caused by destruction of ozone by a number of free radical catalysts—particularly hydroxyl (OH), nitric oxide (NO), atomic chlorine (Cl), and atomic bromine (Br).
At present, most of the OH and NO in the stratosphere are of natural origin, while the concentrations of Cl and Br atoms (classified as "halogen" atoms) have risen through human activity. It appears that the halogen atoms in the stratosphere are formed mainly by the UV-catalyzed breakdown of chlorofluorocarbon (CFC) compounds, commonly called Freons, and bromofluorocarbon compounds, known as Halons, which are transported into the stratosphere after being emitted at the surface.
The free Cl or Br atoms can catalyze the conversion of ozone (O3) to oxygen molecules (O2). The chemical reactions catalyzed by Cl atoms can be written as follows:
- Cl + O3 —> ClO + O2
- ClO + O —> Cl + O2
The overall conversion reaction is:
- O3 + O —> O2 + O2
For this mechanism to operate, there must be a source of O atoms, and these are produced by the breakup of O3 molecules by UV light.
A single chlorine atom could keep on destroying ozone for up to two years (the time scale for transport back down to the troposphere), were it not for reactions that remove Cl from this cycle by forming compounds such as hydrochloric acid. On a per-atom basis, bromine is even more efficient than chlorine at destroying ozone, but there is much less bromine in the atmosphere.
Given that the ozone layer prevents harmful UVC and UVB wavelengths of light from passing through the Earth's atmosphere, observed and projected decreases in ozone have generated worldwide concern. This concern has led to adoption of the Montreal Protocol, which bans the production of CFCs and halons, as well as related ozone-depleting chemicals such as carbon tetrachloride and 1,1,1-trichloroethane (also known as methyl chloroform). It is suspected that increased UV exposure due to ozone depletion may have a variety of biological consequences, including increases in skin cancer, damage to plants, and reduction of plankton populations in the oceans.
Industrial and laboratory production
Industrially, ozone is produced by subjecting oxygen in the air to either (a) short-wavelength UV radiation using a mercury vapor lamp, or (b) a high-voltage electric field in a process called cold discharge or corona discharge. The cold discharge apparatus consists of two metal plates separated by an air gap and an electrical insulator (such as borosilicate glass or mica). When a high-voltage alternating current is applied to the plates, ozone is formed in the air gap, as O2 molecules dissociate and recombine into O3.
In the laboratory, ozone can be produced by electrolysis (electrical breakup) of acidified water. A pencil graphite rod cathode and a platinum wire anode are dipped in a solution containing sulfuric acid (at a concentration of 3 Molar), and the electrodes are connected to a 9-volt battery to generate an electrical current. In the overall reaction, three equivalents of water are converted into one equivalent of ozone and one equivalent of hydrogen. A competing reaction is the formation of oxygen. (See Jorge G. Ibanez et al., 2005, in References below).
Reactions
Ozone is a reagent for many reactions in the laboratory and industry. Some of these are listed here.
Ozone will oxidize metals (except gold, platinum, and iridium) to oxides of the metals in their highest oxidation state. For example, cobalt ions are oxidized from Co2+ to Co3+ as follows:
- 2 Co2+ + 2 H+ + O3 → 2 Co3+ + H2O + O2
Ozone oxidizes oxides to peroxides, or to oxides of higher oxidation number. For example, sulfur dioxide (SO2) is converted to sulfur trioxide (SO3), and nitric oxide (NO) is converted to nitrogen dioxide (NO2), as follows:
- SO2 + O3 → SO3 + O2
- NO + O3 → NO2 + O2
The above reaction is accompanied by chemiluminescence. The NO2 can be further oxidized to NO3:
- NO2 + O3 → NO3 + O2
The NO3 formed can react with NO2 to form N2O5:
- NO2 + NO3 → N2O5
Ozone reacts with carbon to form carbon dioxide, even at room temperature:
- C + 2 O3 → CO2 + 2 O2
Ozone does not react with ammonium salts but it reacts with ammonia (NH3) to form ammonium nitrate (NH4NO3):
- NH3 + 4 O3 → NH4NO3 + 4 O3 + H20
Ozone reacts with sulfides to make sulfates. For instance, lead sulfide (PbS) is converted to lead sulfate (PbSO4):
- PbS + 4 O3 → PbSO4 + 4 O2
Ozone can react with sulfur (S) or sulfur dioxide (SO2) to produce sulfuric acid (H2SO4):
- S + H2O + O3 → H2SO4
- 3 SO2 + 3 H2O + O3 → 3 H2SO4
All three atoms of ozone may also react, as in the reaction with tin(II) chloride (SnCl2) and hydrochloric acid (HCl):
- 3 SnCl2 + 6 HCl + O3 → 3 SnCl4 + 3 H2O
Ozone can be used for combustion reactions, and the burning of gases in ozone produces higher temperatures than burning them in dioxygen (O2). Following is a reaction for the combustion of carbon subnitride (C4N2):
- 3 C4N2 + 4 O3 → 12 CO + 3 N2
Ozone can react at cryogenic (very low) temperatures. At 77 K (-196°C), atomic hydrogen reacts with liquid ozone to form a hydrogen superoxide radical (HO2), which converts to the dimer H2O4 (M. Horvath et al., 1985, pp. 44-49, referenced below):
- H + O3 → HO2 + O
- 2 HO2 → H2O4
It is also possible to form compounds called ozonides, which contain the ozonide anion (O3-). These compounds are explosive and must be stored at cryogenic temperatures. Ozonides for all the alkali metals are known. KO3, RbO3, and CsO3 can be prepared from their respective superoxides. For example, KO3 can be formed from KO2.
- KO2 + O3 → KO3 + O2
NaO3 and LiO3 must be prepared by action of CsO3 in liquid ammonia (NH3) on an ion exchange resin containing Na+ or Li+ ions (Housecroft & Sharpe, 2005, p. 265, referenced below):
- CsO3 + Na+ → Cs+ + NaO3
Ozone can be used to remove manganese (Mn2+) ions from water, by forming a precipitate of MnO(OH)2, which can be filtered:
- 2 Mn2+ + 2 O3 + 4 H2O → 2 MnO(OH)2 (s) + 2 O2 + 4 H+
Ozone will also turn cyanides (CN-) to the 1,000-times less toxic cyanates (CNO-):
- CN- + O3 → CNO- + O2
Finally, ozone will completely decompose urea ((NH2)2CO) (M. Horvath et al., 1985, pp. 259, 269-270, referenced below):
- (NH2)2CO + O3 → N2 + CO2 + 2 H2O
Uses of ozone
Municipal water treatment
Ozone can be used for bleaching materials and killing bacteria. Many municipal drinking water systems kill bacteria with ozone instead of the more common chlorine. Unlike chlorine, ozone does not form organochlorine compounds (which can be harmful), and ozone does not remain in the water after treatment. Some systems introduce a small amount of chlorine to prevent bacterial growth in the pipes, or may use chlorine intermittently, based on the results of periodic testing. Ozone is also popularly used in spas or hot tubs instead of chlorine or bromine to keep the water free of bacteria.
In places where electrical power is abundant, ozone is a cost-effective method of treating water, as it is produced on demand and does not require transportation and storage of hazardous chemicals. Once it has decayed, it leaves no taste or odor in drinking water.
Industrial uses
Industrially, ozone or ozonated water is used for a variety purposes, such as:
- to disinfect water before it is bottled;
- to kill bacteria on food-contact surfaces;
- to scrub yeast and mold spores from the air in food-processing plants;
- to wash fresh fruits and vegetables to kill yeast, mold, and bacteria;
- to chemically attack contaminants in water (iron, arsenic, hydrogen sulfide, nitrites, and complex organics lumped together as "color");
- to provide an aid to flocculation (a process of agglomeration of molecules, which aids in filtration—a process by which iron and arsenic are removed);
- to clean and bleach fabrics (the latter process is patented);
- to assist in processing plastics to allow the adhesion of inks; and
- to age rubber samples when determining the useful life of a batch of rubber.
Medical uses
Ozone has a number of uses in the medical arena. For instance, many hospitals around the world use large ozone generators to decontaminate operating rooms between surgeries. The rooms are cleaned, then sealed airtight and filled with ozone, which effectively kills or neutralizes all remaining bacteria.
Ozone can be used to affect the body's antioxidant-prooxidant balance, because the body usually reacts to its presence by producing antioxidant enzymes. Ozone therapy has blossomed into a thriving field of alternative medicine, and there are a host of claimed applications above and beyond what has actually been verified by studies.
The U.S. Food and Drug Administration (FDA) has not approved the use of ozone therapy on humans. Nonetheless, at least 12 states (AK, AZ, CO, GA, MN, NY, NC, OH, OK, OR, SC and WA) have passed legislation to ensure that alternative therapies are available to consumers. Physicians in those states can legally use ozone as an alternative treatment in their practice, without fear of prosecution. In addition, medical ozone therapy is recognized in Bulgaria, Cuba, Czech Republic, France, Germany, Israel, Italy, Mexico, Romania, and Russia.
At least one death has been attributed to the application of ozone through insufflation in the United States. Nonetheless, "air cleaners" that produce "activated oxygen" (that is, ozone) are often sold.
See also
- Canada-wide standards for particulate matter (PM2.5) and ozone (pdf)
- National Ambient Air Quality Standards (USA)
- Photochemical smog
Further reading
- Greenwood, N. N. and A. Earnshaw. 1997. Chemistry of the Elements (2nd Ed.). Oxford: Butterworth-Heinemann. ISBN 0750633654
ReferencesISBN links support NWE through referral fees
- Bell, Michelle L., Aidan McDermott, Scott L. Zeger, Jonathan M. Samet, Francesca Dominici (2004). "Ozone and Short-term Mortality in 95 US Urban Communities, 1987-2000." Journal of the American Medical Association 292: 2372-2378.
- Ibanez, Jorge G., Rodrigo Mayen-Mondragon, and M. T. Moran-Moran: "Laboratory Experiments on the Electrochemical Remediation of the Environment. Part 7: Microscale Production of Ozone," J. Chem. Ed. 82:10 (October 2005): 1546 Abstract.
- Horvath, M., L. Bilitzky, & J. Huttner. 1985. "Ozone."
- Housecroft & Sharpe. 2005. "Inorganic Chemistry."
- Seinfeld, John H., and Spyros N. Pandis. 1998. Atmospheric Chemistry and Physics - From Air Pollution to Climate Change. John Wiley and Sons, Inc. ISBN 0471178160
- Wayne, Richard P. 2000. Chemistry of Atmospheres (3rd Ed.). Oxford University Press. ISBN 0-19-850375-X
External links
All links retrieved November 18, 2022.
- NIOSH Pocket Guide to Chemical Hazards
- Air Pollution Ozone
- U.S. Environmental Protection Agency Live Ozone Map
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.