Difference between revisions of "Nucleotide" - New World Encyclopedia
| Line 7: | Line 7: | ||
*Nucleotides also serve as regulators of metabolism. Cyclic AMP is a ubiquitous mediator of the action of many hormones. Covalent modifications introduced by ATP alter the activities of many enzymes (e.g., phosphorylation of glycogen synthase) | *Nucleotides also serve as regulators of metabolism. Cyclic AMP is a ubiquitous mediator of the action of many hormones. Covalent modifications introduced by ATP alter the activities of many enzymes (e.g., phosphorylation of glycogen synthase) | ||
| − | perhaps explain reason for ubiquity of ribonucleotides in particular—evolutionarily, RNA is believed to have come before DNA and proteins. (Stryer 115 and ch. 17) (also see rna-world hypothesis in wikipedia) | + | perhaps explain reason for ubiquity of ribonucleotides in particular—evolutionarily, RNA is believed to have come before DNA and proteins. (Stryer 115 and ch. 17) (also see rna-world hypothesis in wikipedia)Discovery that certain RNA molecules, in addition to proteins, can also have enzymatic function (ribozymes). Early RNA might have first catalyzed their own replication and developed a range of enzymatic activities. Next, RNA molecules began to synthesize proteins which are more versatile (with their 20 standard amino acid components with unique side chains) than the four bases of nucleotides. Next, DNA might have been formed by reverse transcription of RNA, with DNA eventually replacing RNA as the storage form of genetic material because of its double helix is a more stable and dependable structure. These common strategies/shared themes of metabolism suggest the interconnectedness of life and its common origins. |
==Chemical structure and nomenclature== | ==Chemical structure and nomenclature== | ||
Revision as of 20:40, 17 August 2006
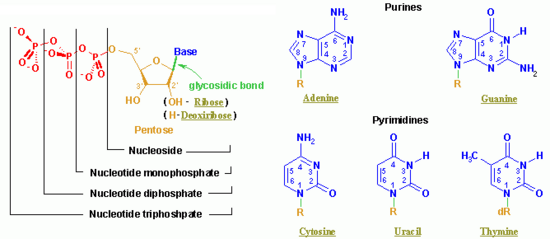
A nucleotide is a chemical compound with three components: a nitrogen-containing base, a pentose (five-carbon) sugar, and one or more phosphate groups. Although best known as the structural units of the nucleic acids DNA and RNA, which encode genetic information in organisms, nucleotides participate in nearly all biochemical processes.
Nucleotides' role in metabolism:
- ATP, an adenine nucleotide, is a universal energy currency of energy in biological systems.
- Adenine nucleotides are components of three major coenzymes, NAD plus, FAD, and CoA
- Nucleotides also serve as regulators of metabolism. Cyclic AMP is a ubiquitous mediator of the action of many hormones. Covalent modifications introduced by ATP alter the activities of many enzymes (e.g., phosphorylation of glycogen synthase)
perhaps explain reason for ubiquity of ribonucleotides in particular—evolutionarily, RNA is believed to have come before DNA and proteins. (Stryer 115 and ch. 17) (also see rna-world hypothesis in wikipedia)Discovery that certain RNA molecules, in addition to proteins, can also have enzymatic function (ribozymes). Early RNA might have first catalyzed their own replication and developed a range of enzymatic activities. Next, RNA molecules began to synthesize proteins which are more versatile (with their 20 standard amino acid components with unique side chains) than the four bases of nucleotides. Next, DNA might have been formed by reverse transcription of RNA, with DNA eventually replacing RNA as the storage form of genetic material because of its double helix is a more stable and dependable structure. These common strategies/shared themes of metabolism suggest the interconnectedness of life and its common origins.
Chemical structure and nomenclature
The nitrogen-containing base of a nucleotide (also called the nucleobase) is typically a derivative of either purine or pyrimidine, which are heterocylic compounds, or organic compounds that contain a ring structure with atoms in addition to carbo (such as sulfur, oxygen or nitrogen). The most common bases in nucleotides are:
The sugar component is either deoxyribose or ribose. (“Deoxy” simply indicates that the sugar lacks an oxygen atom present in ribose, the parent compound.) Depending on their base sugar, nucleotides are therefore known as “deoxyribonucleotides” or “ribonucleotides.” The nucleic acid DNA (which stands for deoxyribonucleic acid) is built of nucleotides with a deoxyribose sugar, whereas RNA (or ribonucleic acid) contains nucleotides composed of ribose sugars.
Nucleotide names are abbreviated into standard three- or four-letter codes that indicate their structural components:
- The first letter is lower case and indicates whether the nucleotide in question is a deoxyribonucleotide (denoted by a d) or a ribonucleotide (no letter).
- The second letter indicates the nucleoside corresponding to the base. Nucleosides resemble the structure of nucleotides (i.e., they contain a purine or pyrimidine base bonded to a sugar) but lack the phosphate group. A nucleotide can thus also be defined as the phosphate ester of a nucleotide. In chemistry, esters are organic compounds in which an organic group replaces a hydrogen atom (or more than one) in an oxygen acid. The abbreviations are as follows:
- G: Guanine
- A: Adenine
- T: Thymine
- C: Cytosine
- U: Uracil (which is not present in DNA, but takes the place of thymine in RNA)
- The third and fourth letters indicate the length of the attached phosphate chain (Mono-, Di-, Tri-) and the presence of a phosphate (P).
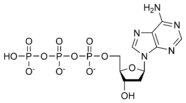
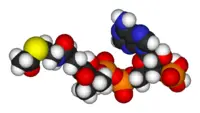
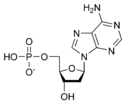
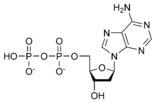
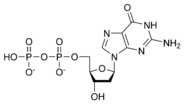
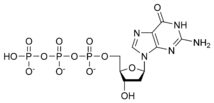
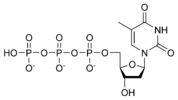
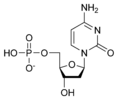
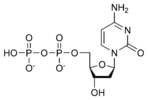
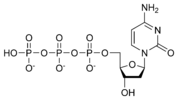
Thus, for example, deoxy-adenosine-triphosphate (pictured at right), one of the activated precursors in the synthesis of DNA, is abbreviated as dATP.
Nucleotides encode genetic information
DNA is a polymer of deoxyribonucleotide units. Polymer is a term used to describe molecules consisting of structural units and a large number of repeating units connected by covalent chemical bonds.
Structure of DNA: two helical polynucleotide chains are coiled around a common axis. The chains run in opposite directions. The bases are on the inside of the helix, while the sugar-phosphate backbone is on the outside. The two chains are held together by hydrogen bonds between pairs of bases. Adenine is always paired with thymine; guanine with cytosine (i.e., a purine to a pyrimidine).
Although sometimes called "the molecule of heredity", DNA macromolecules as people typically think of them are not single molecules. Rather, they are pairs of molecules, which entwine like vines to form a double helix (see the illustration below). Each vine-like molecule is a strand of DNA, a chemically linked chain of nucleotides.
Because pairing causes the nucleotide bases to face the helical axis, the sugar and phosphate groups of the nucleotides run along the outside; the two chains they form are sometimes called the "backbones" of the helix. In fact, it is chemical bonds between the phosphates and the sugars that link one nucleotide to the next in the DNA strand.
In DNA, bases carry genetic information while sugar and phosphate groups provide a structural role (as the base is the variable part). Within a gene, the sequence of nucleotides along a DNA strand defines a messenger RNA sequence which then defines a protein, that an organism is liable to manufacture or "express" at one or several points in its life using the information of the sequence. The relationship between the nucleotide sequence and the amino-acid sequence of the protein is determined by simple cellular rules of translation, known collectively as the genetic code. The genetic code is the relation between the sequence of bases in DNA (or its RNA transcript) and the sequence of amino acids in proteins. Amino acids are coded by groups of three bases (called codons) starting from a fixed point. It consists of three-letter 'words' (termed a codon) formed from a sequence of three nucleotides (e.g. ACT, CAG, TTT). These codons can then be translated with messenger RNA and then transfer RNA, with a codon corresponding to a particular amino acid.
Two differences of RNA, which can serve as genetic material in viruses: (1) sugar units are riboses rather than deoxyriboses and (2) one of the four major bases is uracil (U) instead of thymine (T); can be single or double-stranded
RNA molecules can contain as few as 75 nucleotides to more than 5000 nucleotides.
Nucleotides function in cell metabolism
ATP is the universal energy currency of the cell
While ATP (adenosine triphosphate) is one of four nucleotides required for the synthesis of ribonucleic acids, it is primarily known in biochemistry for its role in metabolism as the "molecular currency" of intracellular energy transfer. As the name suggests, the structure of this molecule consists of a purine base (adenine), a ribose sugar, and three phosphate groups.
The energy in phosphate bonds is key. ATP energy is released when hydrolysis of the phosphate-phosphate bonds is carried out. This energy can be used to power reactions such the active transport of molecules across cell membranes, the synthesis of macromolecules (Eg. proteins), muscle contractions, endocytosis, and exocytosis.as Also, the hydrolysis yields free inorganic Pi and ADP, which can be broken down further to another Pi and AMP. ATP can also be broken down to AMP directly, with the formation of PPi. This last reaction has the advantage of being an effectively irreversible process in aqueous solution.
At any given moment, the total quantity of ATP in the human body is about 0.1 mole. The energy used by human cells requires the hydrolysis of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2000 to 3000 times during a single day. ATP cannot be stored, hence its consumption must closely follow its synthesis.
- Note that ATP isn't usually actually synthethised from scratch, but simply recharged from ADP (Adenosine Diphosphate). The amount of ATP + ADP in your body remains roughly the same, it just keeps cycling.
ATP can be produced by various cellular processes: Under aerobic conditions, the majority of the synthesis occurs in mitochondria during oxidative phosphorylation and is catalyzed by ATP synthase and, to a lesser degree, under anaerobic conditions by fermentation.
Other nucleotide triphosphates that have high-energy phosphate bonds may also power some biosynthetic reactions: namely, guanosine triphosphate (GTP), uradine triphosphate (UTP), and cytidine triphosphate (CTP).
Several nucleotides function as coenzymes
Define cofactor/coenzyme; explain that they are regenerated. Recurring set of carriers that faciliate the reactions of metabolism:
- (NAD+) (Nicotinamide adenine dinucleotide) and NADP (nicotinamide adenine dinucleotide phosphate) are two important cofactors found in cells. NADH is the reduced form of NAD+, and NAD+ is the oxidized form of NADH. It forms NADP with the addition of a phosphate group to the 2' position of the adenosyl nucleotide through an ester linkage. The reactive part is nicotinamide, a derivative of pyridine.
NAD is used extensively in glycolysis and the citric acid cycle of cellular respiration. The reducing potential (ability to donate electrons) stored in NADH can be converted to ATP through the electron transport chain or used for anabolic metabolism. [explain role as carrier molecule]
NADP is used in anabolic reactions, such as fatty acid and nucleic acid synthesis, that require NADPH as a reducing agent. In chloroplasts, NADP is an oxidizing agent important in the preliminary reactions of photosynthesis. The NADPH produced by photosynthesis is then used as reducing power for the biosynthetic reactions in the Calvin cycle of photosynthesis.
The other major electron carrier in the oxiation of fuel molecules is FAD (flavin adenine dinucleotide).
- Coenzyme A (CoA) is a coenzyme, notable for its role in the synthesis and oxidization of fatty acids, and the oxidation of pyruvate in the citric acid cycle.
The main function of coenzyme A is to carry acyl groups (such as the acetyl group) or thioesters. A molecule of coenzyme A carrying an acetyl group is also referred to as acetyl-CoA. (A stands for acetylation). Acetyl CoA has a high acetyl group-transfer potential, meaning that it carries an activated acetyl group, which it can deliver for degradation and energy generation, or for biosynthesis. For example, some proteins acquire long-chain fatty acyl groups to enable them to anchor into bilayer membranes.
Acetyl-CoA is an important molecule itself. It is the precursor to HMG CoA, which is a vital component in cholesterol and ketone synthesis. Its main task is conveying the carbon atoms within the acetyl group to the citric acid cycle to be oxidized for energy production.
Nucleotides also play a role in signaling
Cyclic adenosine monophosphate (cAMP or cyclic AMP) is a molecule that is important in many biological processes; it is derived from adenosine triphosphate (ATP). cAMP controls many biological processes, including glycogen decomposition into glucose (glycogenolysis), and lipolysis. cAMP is a second messenger (within a cell), used for intracellular signal transduction, such as transferring the effects of hormones like glucagon and adrenaline, which are first messengers (from one cell to another) and cannot get through the cell membrane. How does cAMP achieve this effect? Most of these effects of cyclic AMP in eukaryotic cells are mediated by its activation of a specific protein kinase called PKA (protein kinase A). Cyclic AMP binds to specific locations on the two regulatory units of the protein kinase, and causes dissociation between the regulatory and catalytic subunits, thus activating the catalytic units and enabling them to phosphorylate substrate proteins.
Chemical structures
Nucleotides
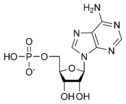 Adenosine monophosphate AMP |
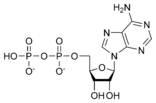 Adenosine diphosphate ADP |
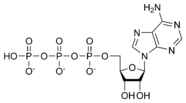 Adenosine triphosphate ATP |
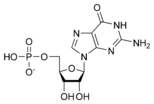 Guanosine monophosphate GMP |
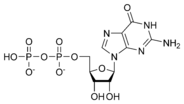 Guanosine diphosphate GDP |
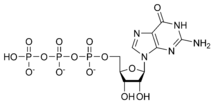 Guanosine triphosphate GTP |
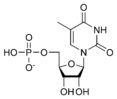 Thymidine monophosphate TMP |
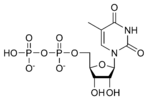 Thymidine diphosphate TDP |
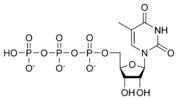 Thymidine triphosphate TTP |
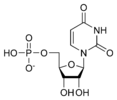 Uridine monophosphate UMP |
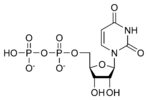 Uridine diphosphate UDP |
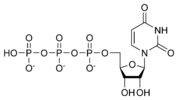 Uridine triphosphate UTP |
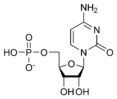 Cytidine monophosphate CMP |
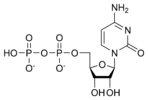 Cytidine diphosphate CDP |
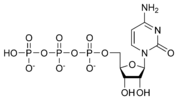 Cytidine triphosphate CTP |
Deoxynucleotides
| Code | Equivalence | Complement |
|---|---|---|
| A | A | T or U |
| C | C | G |
| G | G | C |
| T or U | T | A |
| M | A or C | K |
| R | A or G | Y |
| W | A or T | W |
| S | C or G | S |
| Y | C or T | R |
| K | G or T | M |
| V | A or C or G | B |
| H | A or C or T | D |
| D | A or G or T | H |
| B | C or G or T | V |
| X or N | A or C or G or T | X |
ReferencesISBN links support NWE through referral fees
- Stryer, Lubert. 1995. Biochemistry, 4th edition. New York, NY: W.H. Freeman.
External links
- Abbreviations and Symbols for Nucleic Acids, Polynucleotides and their Constituents (IUPAC)
- Provisional Recommendations 2004 (IUPAC)
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - mtDNA - Oligonucleotide |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.