Difference between revisions of "Histidine" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 10: | Line 10: | ||
}} | }} | ||
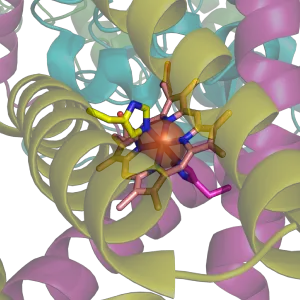
[[Image:Succinate Dehygrogenase 1YQ3 Haem group.png|thumb|right|The histidine bound [[heme]] group of [[succinate dehydrogenase]], an [[electron carrier]] in the [[mitochondria]]l [[electron transfer chain]]. The large semi-transparent sphere indicates the location of the [[iron]] [[ion]]. From {{PDB|1YQ3}}.]] | [[Image:Succinate Dehygrogenase 1YQ3 Haem group.png|thumb|right|The histidine bound [[heme]] group of [[succinate dehydrogenase]], an [[electron carrier]] in the [[mitochondria]]l [[electron transfer chain]]. The large semi-transparent sphere indicates the location of the [[iron]] [[ion]]. From {{PDB|1YQ3}}.]] | ||
| − | '''Histidine''' | + | '''Histidine''' is an α-[[amino acid]] that is common in many proteins and is essential in the human diet, at least in children. It is the precusor of [[histamine]] and important in the synthesis of [[purine]]s. Like argenine and lysine, histidine is a basic amino acid. In even slightly acidic conditions, protonation of the [[nitrogen]] occurs, changing the properties of histidine and the polypeptide as a whole. It is used in many proteins as a regulatory mechanism, changing the conformation and behavior of the polypeptide in acidic regions such as the late endosome or lysosome, enforcing conformation change in [[enzyme]]s. |
| + | |||
| + | The L-isomer of histidine, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] common in animal proteins and required for normal functioning in humans. Histidine is variously classified as a "conditionally essential" or "essential" amino acid. An [[amino acid#essential amino acid|essential amino acid]] is one that cannot be synthesized by the [[human body]] from other compounds through chemical reactions, or at a rate sufficient to meet the body's physiological needs, and thus has to be obtained from the diet. Infants are unable to effectively synthesize histidine, making it nutritionally essential for infants. Historically, it has been considered non-essential in adults, who can go for periods of time without it in the diet, but it also is commonly considered essential for adults as well. | ||
| + | |||
| + | Histidine three letter code is His, its one letter code is H, and its systematic name is 2-Amino-3-(1H-imidazol-4-yl)- | ||
| + | propanoic acid (IUPAC-IUB 1983). | ||
| + | |||
| + | |||
| + | ==Structure and chemical properties== | ||
| + | In [[biochemistry]], the term [[amino acid]] is frequently used to refer specifically to ''alpha amino acids'': those amino acids in which the amino and carboxylate groups are attached to the same [[carbon]], the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is: | ||
| + | |||
| + | ''R'' | ||
| + | | | ||
| + | H<sub>2</sub>N-C-COOH | ||
| + | | | ||
| + | H | ||
| + | where ''R'' represents a ''side chain'' specific to each amino acid. The exception to this basic structure is [[proline]], whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group. | ||
| + | |||
| + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. There are two enantiomers of histidine, D-histidine and L-histidine, but only the L-stereoisomer is involved in synthesis of [[mammal]]ian proteins. | ||
| + | |||
| + | Histidine's general chemical formula is C<sub>6</sub>H<sub>9</sub>N<sub>3</sub>O<sub>2</sub>(IUPAC-IUB 1983). | ||
| − | |||
The [[imidazole]] [[side chains]] and the relatively neutral pKa of histidine (ca 6.0) mean that relatively small shifts in cellular [[pH]] will change its charge. For this reason, this amino acid side chain finds its way into considerable use as a co-ordinating [[ligand]] in [[metalloprotein]]s, and also as a [[catalyst|catalytic]] site in certain [[enzyme]]s. The imidazole side chain has two nitrogens with different properties: One is bound to hydrogen and donates its lone pair to the aromatic ring and as such is slighty [[acidic]], whereas the other one donates only one electron pair to the ring so it has a free lone pair and is [[basic (chemistry)|basic]]. These properties are exploited in different ways in proteins. In [[catalytic triad]]s, the basic nitrogen of histidine is used to abstract a proton from [[serine]], [[threonine]] or [[cysteine]] to activate it as a [[nucleophile]]. In a histidine proton shuttle, histidine is used to quickly shuttle protons, it can do this by abstracting a proton with its basic nitrogen to make a positively-charged intermediate and then use another molecule, a buffer, to extract the proton from its acidic nitrogen. In [[carbonic anhydrase]]s, a histidine proton shuttle is utilized to rapidly shuttle protons away from a zinc-bound water molecule to quickly regenerate the active form of the enzyme. | The [[imidazole]] [[side chains]] and the relatively neutral pKa of histidine (ca 6.0) mean that relatively small shifts in cellular [[pH]] will change its charge. For this reason, this amino acid side chain finds its way into considerable use as a co-ordinating [[ligand]] in [[metalloprotein]]s, and also as a [[catalyst|catalytic]] site in certain [[enzyme]]s. The imidazole side chain has two nitrogens with different properties: One is bound to hydrogen and donates its lone pair to the aromatic ring and as such is slighty [[acidic]], whereas the other one donates only one electron pair to the ring so it has a free lone pair and is [[basic (chemistry)|basic]]. These properties are exploited in different ways in proteins. In [[catalytic triad]]s, the basic nitrogen of histidine is used to abstract a proton from [[serine]], [[threonine]] or [[cysteine]] to activate it as a [[nucleophile]]. In a histidine proton shuttle, histidine is used to quickly shuttle protons, it can do this by abstracting a proton with its basic nitrogen to make a positively-charged intermediate and then use another molecule, a buffer, to extract the proton from its acidic nitrogen. In [[carbonic anhydrase]]s, a histidine proton shuttle is utilized to rapidly shuttle protons away from a zinc-bound water molecule to quickly regenerate the active form of the enzyme. | ||
| Line 35: | Line 54: | ||
Image:Histidine_resonant.png|Histidine | Image:Histidine_resonant.png|Histidine | ||
</gallery> | </gallery> | ||
| + | |||
| + | ==References== | ||
| + | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319. | ||
| + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | ||
| + | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry'', 3rd ed. New York: Worth Publishing. ISBN 1572591536. | ||
| + | |||
== External links == | == External links == | ||
Revision as of 23:49, 25 June 2007
| |
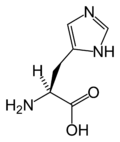
Histidine | |
| Systematic (IUPAC) name | |
| 2-amino-3-(3H-imidazol-4-yl)propanoic acid | |
| Identifiers | |
| CAS number | 71-00-1 |
| PubChem | 773 |
| Chemical data | |
| Formula | C6H9N3O2 |
| Mol. weight | 155.16 |
| SMILES | N[C@@H](Cc1[nH]cnc1)C(O)=O |
| Complete data | |
Histidine is an α-amino acid that is common in many proteins and is essential in the human diet, at least in children. It is the precusor of histamine and important in the synthesis of purines. Like argenine and lysine, histidine is a basic amino acid. In even slightly acidic conditions, protonation of the nitrogen occurs, changing the properties of histidine and the polypeptide as a whole. It is used in many proteins as a regulatory mechanism, changing the conformation and behavior of the polypeptide in acidic regions such as the late endosome or lysosome, enforcing conformation change in enzymes.
The L-isomer of histidine, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids common in animal proteins and required for normal functioning in humans. Histidine is variously classified as a "conditionally essential" or "essential" amino acid. An essential amino acid is one that cannot be synthesized by the human body from other compounds through chemical reactions, or at a rate sufficient to meet the body's physiological needs, and thus has to be obtained from the diet. Infants are unable to effectively synthesize histidine, making it nutritionally essential for infants. Historically, it has been considered non-essential in adults, who can go for periods of time without it in the diet, but it also is commonly considered essential for adults as well.
Histidine three letter code is His, its one letter code is H, and its systematic name is 2-Amino-3-(1H-imidazol-4-yl)- propanoic acid (IUPAC-IUB 1983).
Structure and chemical properties
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid. The exception to this basic structure is proline, whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. There are two enantiomers of histidine, D-histidine and L-histidine, but only the L-stereoisomer is involved in synthesis of mammalian proteins.
Histidine's general chemical formula is C6H9N3O2(IUPAC-IUB 1983).
The imidazole side chains and the relatively neutral pKa of histidine (ca 6.0) mean that relatively small shifts in cellular pH will change its charge. For this reason, this amino acid side chain finds its way into considerable use as a co-ordinating ligand in metalloproteins, and also as a catalytic site in certain enzymes. The imidazole side chain has two nitrogens with different properties: One is bound to hydrogen and donates its lone pair to the aromatic ring and as such is slighty acidic, whereas the other one donates only one electron pair to the ring so it has a free lone pair and is basic. These properties are exploited in different ways in proteins. In catalytic triads, the basic nitrogen of histidine is used to abstract a proton from serine, threonine or cysteine to activate it as a nucleophile. In a histidine proton shuttle, histidine is used to quickly shuttle protons, it can do this by abstracting a proton with its basic nitrogen to make a positively-charged intermediate and then use another molecule, a buffer, to extract the proton from its acidic nitrogen. In carbonic anhydrases, a histidine proton shuttle is utilized to rapidly shuttle protons away from a zinc-bound water molecule to quickly regenerate the active form of the enzyme.
Metabolism
The amino acid is a precursor for histamine and carnosine biosynthesis.
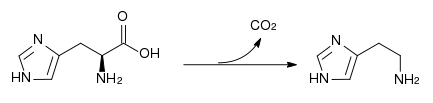
The enzyme histidine ammonia-lyase converts histidine into ammonia and urocanic acid. A deficiency in this enzyme is present in the rare metabolic disorder histidinemia.
Sources
Histidine is found in fruits such as bananas and grapes, meat and poultry, and milk and milk products. It is also found in root vegetables and all green vegetables, though in lesser quantities.
Forms
There are two enantiomers: D-histidine and L-histidine.
History
Histidine was first isolated in 1896 by German physician Albrecht Kossel.
Additional images
ReferencesISBN links support NWE through referral fees
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
External links
- Histidine biosynthesis (early stages)
- Histidine biosynthesis (later stages)
- Histidine catabolism
- Computational Chemistry Wiki
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
