Difference between revisions of "Threonine" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 49: | Line 49: | ||
The threonine residue is susceptible to numerous [[posttranslational modification]]s. The hydroxy [[side chain]] can undergo O-linked [[glycosylation]]. Additionally, threonine residues undergo [[phosphorylation]] through the action of a threonine [[kinase]]. In its phosphorylated form, it can be referred to as phosphothreonine. | The threonine residue is susceptible to numerous [[posttranslational modification]]s. The hydroxy [[side chain]] can undergo O-linked [[glycosylation]]. Additionally, threonine residues undergo [[phosphorylation]] through the action of a threonine [[kinase]]. In its phosphorylated form, it can be referred to as phosphothreonine. | ||
| + | |||
| + | '''Serine''' is an α-[[amino acid]] that is common in many [[protein]]s, sometimes in substantial concentrations in the outer regions of soluble proteins due to its hydrophilic nature. Serine is an important component of phospholipids and participates in the biosynthesis of [[purine]]s and [[pyriminidine]]s, as well as such amino acids as [[cysteine]] and [[glycine]]. With an easily removed hydrogen on the hydroxyl side chain, serine is often a hydrogen donor in enzymes, such as trypsin and chymotrypsin, playing an important role in their function as catalysts. | ||
| + | |||
| + | In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] required for normal functioning. However, it is considered to be a [[amino acid#essential amino acids|"non-essential"]] amino acid since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. | ||
| + | |||
| + | Human creativity, which can be used for good or ill purposes, has exploited serine's role in the active site of the enzyme acetylcholine esterase to produce both nerve gases, such as Sarin that causes painful deaths in humans, and insecticides, which are designed to increase human agricultural productivity and prosperity. (See [[#Function|function]] below.) | ||
| + | |||
| + | Serine's three letter code is Ser, its one letter code is S, its codons are AGU and AGC, and its systematic name is 2-Amino-3-hydroxypropanoic acid (IUPAC-IUB 1983). The name serine was derived from the Latin for silk, "sericum," since serine was first isolated from silk protein. While the amino acids [[glycine]] and [[alanine]] make up the bulk of silk protein, it is also a rich source of serine. | ||
| + | |||
| + | Threonine Thr T 2-Amino-3-hydroxybutanoic | ||
| + | acid e CH3-CH(OH)-CH(NH2)-COOH | ||
| + | |||
| + | ==Structure== | ||
| + | In [[biochemistry]], the term [[amino acid]] is frequently used to refer specifically to ''alpha amino acids'': those amino acids in which the amino and carboxylate groups are attached to the same [[carbon]], the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is: | ||
| + | |||
| + | ''R'' | ||
| + | | | ||
| + | H<sub>2</sub>N-C-COOH | ||
| + | | | ||
| + | H | ||
| + | where ''R'' represents a ''side chain'' specific to each amino acid. | ||
| + | |||
| + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In serine, only the L-stereoisomer is involved in synthesis of [[mammal]]ian proteins. | ||
| + | |||
| + | Serine has the chemical formula HO-CH<sub>2</sub>-CH(NH<sub>2</sub>)-COOH (alternatively, HO<sub>2</sub>C-CH(NH<sub>2</sub>)-CH<sub>2</sub>-OH), or more generally, C<sub>3</sub>H<sub>7</sub>NO<sub>3</sub>. | ||
| + | |||
| + | Threonine Thr T 2-Amino-3-hydroxybutanoic | ||
| + | acid e CH3-CH(OH)-CH(NH2)-COOH | ||
| + | |||
| + | Serine, like [[threonine]], has a short group ended with a hydroxyl group. The hydroxyl group attached makes it a polar amino acid. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in [[enzyme]]s. Both are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them. | ||
| + | |||
| + | Behaves similarly to serine. | ||
====Allo-threonine==== | ====Allo-threonine==== | ||
| Line 76: | Line 108: | ||
==References== | ==References== | ||
<references/> | <references/> | ||
| + | |||
| + | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319. | ||
| + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | ||
| + | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry'', 3rd ed. New York: Worth Publishing. ISBN 1572591536. | ||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
| + | |||
== External links == | == External links == | ||
*[http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/Thr.html Threonine biosynthesis] | *[http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/Thr.html Threonine biosynthesis] | ||
Revision as of 23:55, 27 June 2007
| Threonine | |
|---|---|
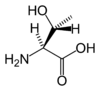
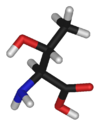
| Systematic name | (2S,3R)-2-Amino- 3-hydroxybutanoic acid |
| Abbreviations | Thr T |
| Chemical formula | C4H9NO3 |
| Molecular mass | 119.12 g mol-1 |
| Melting point | 256 °C |
| Density | ? g cm-3 |
| Isoelectric point | 5.60 |
| pKa | 2.20 8.96 |
| PubChem | 6288 |
| CAS number | [72-19-5] |
| EINECS number | 200-774-1 |
| SMILES | C[C@@H](O)[C@H](N)C(O)=O |
| |
| Disclaimer and references | |
Threonine is an α-amino acid with the chemical formula HO2CCH(NH2)CH(OH)CH3. Its three letter code is thr, its one letter code is T, and its codons are ACU and ACA. This essential amino acid is classified as polar. Together with serine and tyrosine, threonine is one of three proteinogenic amino acids bearing an alcohol group.
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxy side chain can undergo O-linked glycosylation. Additionally, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine.
Serine is an α-amino acid that is common in many proteins, sometimes in substantial concentrations in the outer regions of soluble proteins due to its hydrophilic nature. Serine is an important component of phospholipids and participates in the biosynthesis of purines and pyriminidines, as well as such amino acids as cysteine and glycine. With an easily removed hydrogen on the hydroxyl side chain, serine is often a hydrogen donor in enzymes, such as trypsin and chymotrypsin, playing an important role in their function as catalysts.
In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids required for normal functioning. However, it is considered to be a "non-essential" amino acid since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions.
Human creativity, which can be used for good or ill purposes, has exploited serine's role in the active site of the enzyme acetylcholine esterase to produce both nerve gases, such as Sarin that causes painful deaths in humans, and insecticides, which are designed to increase human agricultural productivity and prosperity. (See function below.)
Serine's three letter code is Ser, its one letter code is S, its codons are AGU and AGC, and its systematic name is 2-Amino-3-hydroxypropanoic acid (IUPAC-IUB 1983). The name serine was derived from the Latin for silk, "sericum," since serine was first isolated from silk protein. While the amino acids glycine and alanine make up the bulk of silk protein, it is also a rich source of serine.
Threonine Thr T 2-Amino-3-hydroxybutanoic acid e CH3-CH(OH)-CH(NH2)-COOH
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In serine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Serine has the chemical formula HO-CH2-CH(NH2)-COOH (alternatively, HO2C-CH(NH2)-CH2-OH), or more generally, C3H7NO3.
Threonine Thr T 2-Amino-3-hydroxybutanoic acid e CH3-CH(OH)-CH(NH2)-COOH
Serine, like threonine, has a short group ended with a hydroxyl group. The hydroxyl group attached makes it a polar amino acid. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in enzymes. Both are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them.
Behaves similarly to serine.
Allo-threonine
With two chiral centers, threonine can exist in four possible stereoisomers, or two possible diastereomers of L-threonine. However, the name L-threonine is used for one single enantiomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The second diastereomer (2S,3S), which is rarely present in nature, is called L-allo-threonine.
Biosynthesis
As an essential amino acid, threonine is not synthesized in humans, hence we must ingest threonine or, more commonly, threonine-containing proteins. In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group.[1] Enzymes involved in a typical biosynthesis of threonine include:
- aspartokinase
- α-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine kinase
- threonine synthase
Metabolism
Threonine is metabolized in two ways:
- It is converted to pyruvate
- It is converted to alpha-ketobutyrate, and thereby enter the pathway leading to succinyl CoA.
Synthesis
Racemic threonine can be prepared from crotonic acid by alpha-functionalization using mercury(II) acetate.[2]
Sources
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, and sesame seeds.[citation needed]
ReferencesISBN links support NWE through referral fees
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Carter, H. E.; West, H. D. (1955). "dl-Threonine". Org. Synth.; Coll. Vol. 3: 813.
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.