Difference between revisions of "Proline" - New World Encyclopedia
({{Contracted}}) |
Rick Swarts (talk | contribs) |
||
| Line 29: | Line 29: | ||
'''L-Proline''' is one of the twenty [[proteinogenic]] units which are used in living organisms as the building blocks of [[protein]]s. The other nineteen units are all primary [[amino acid]]s, but due to the cyclic binding of the three-[[carbon]] [[side chain]] to the [[nitrogen]] of the backbone, proline lacks a primary [[amine]] group (−NH<sub>2</sub>). The nitrogen in proline is properly referred to as a secondary [[amine]]. Proline is sometimes called an [[imino acid]], although the [[IUPAC]] definition of an [[imine]] requires a carbon-nitrogen [[double bond]]. In biological terminology, however, the category "amino acids" is generally taken to include proline. | '''L-Proline''' is one of the twenty [[proteinogenic]] units which are used in living organisms as the building blocks of [[protein]]s. The other nineteen units are all primary [[amino acid]]s, but due to the cyclic binding of the three-[[carbon]] [[side chain]] to the [[nitrogen]] of the backbone, proline lacks a primary [[amine]] group (−NH<sub>2</sub>). The nitrogen in proline is properly referred to as a secondary [[amine]]. Proline is sometimes called an [[imino acid]], although the [[IUPAC]] definition of an [[imine]] requires a carbon-nitrogen [[double bond]]. In biological terminology, however, the category "amino acids" is generally taken to include proline. | ||
| − | == | + | '''Serine''' is an α-[[amino acid]] that is common in many [[protein]]s, sometimes in substantial concentrations in the outer regions of soluble proteins due to its hydrophilic nature. Serine is an important component of phospholipids and participates in the biosynthesis of [[purine]]s and [[pyriminidine]]s, as well as such amino acids as [[cysteine]] and [[glycine]]. With an easily removed hydrogen on the hydroxyl side chain, serine is often a hydrogen donor in enzymes, such as trypsin and chymotrypsin, playing an important role in their function as catalysts. |
| + | |||
| + | In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 [[amino acid#standard amino acid|standard amino acids]] required for normal functioning. However, it is considered to be a [[amino acid#essential amino acids|"non-essential"]] amino acid since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions. | ||
| + | |||
| + | Human creativity, which can be used for good or ill purposes, has exploited serine's role in the active site of the enzyme acetylcholine esterase to produce both nerve gases, such as Sarin that causes painful deaths in humans, and insecticides, which are designed to increase human agricultural productivity and prosperity. (See [[#Function|function]] below.) | ||
| + | |||
| + | Serine's three letter code is Ser, its one letter code is S, its codons are AGU and AGC, and its systematic name is 2-Amino-3-hydroxypropanoic acid (IUPAC-IUB 1983). The name serine was derived from the Latin for silk, "sericum," since serine was first isolated from silk protein. While the amino acids [[glycine]] and [[alanine]] make up the bulk of silk protein, it is also a rich source of serine. | ||
| + | |||
| + | Proline Pro P Pyrrolidine-2-carboxylic acid | ||
| + | |||
| + | ==Structure== | ||
| + | In [[biochemistry]], the term [[amino acid]] is frequently used to refer specifically to ''alpha amino acids'': those amino acids in which the amino and carboxylate groups are attached to the same [[carbon]], the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is: | ||
| + | |||
| + | ''R'' | ||
| + | | | ||
| + | H<sub>2</sub>N-C-COOH | ||
| + | | | ||
| + | H | ||
| + | where ''R'' represents a ''side chain'' specific to each amino acid. The exception to this basic structure is [[proline]], whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group. | ||
| + | |||
| + | Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in [[protein]]s. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In serine, only the L-stereoisomer is involved in synthesis of [[mammal]]ian proteins. | ||
| + | |||
| + | Serine has the chemical formula HO-CH<sub>2</sub>-CH(NH<sub>2</sub>)-COOH (alternatively, HO<sub>2</sub>C-CH(NH<sub>2</sub>)-CH<sub>2</sub>-OH), or more generally, C<sub>3</sub>H<sub>7</sub>NO<sub>3</sub>. | ||
| + | |||
| + | Serine, like [[threonine]], has a short group ended with a hydroxyl group. The hydroxyl group attached makes it a polar amino acid. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in [[enzyme]]s. Both are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them. | ||
| + | |||
The distinctive cyclic structure of proline's side chain locks its <math>\phi</math> backbone [[dihedral angle]] at approximately -75°, giving proline an exceptional conformational rigidity compared to other amino acids. Hence, proline loses less conformational entropy upon folding, which may account for its higher prevalence in the proteins of thermophilic organisms. | The distinctive cyclic structure of proline's side chain locks its <math>\phi</math> backbone [[dihedral angle]] at approximately -75°, giving proline an exceptional conformational rigidity compared to other amino acids. Hence, proline loses less conformational entropy upon folding, which may account for its higher prevalence in the proteins of thermophilic organisms. | ||
| Line 70: | Line 95: | ||
==References== | ==References== | ||
| − | + | ||
* Balbach J, Schmid FX. (2000). Proline isomerization and its catalysis in protein folding. In ''Mechanisms of Protein Folding'' 2nd ed. Editor RH Pain. Oxford University Press. | * Balbach J, Schmid FX. (2000). Proline isomerization and its catalysis in protein folding. In ''Mechanisms of Protein Folding'' 2nd ed. Editor RH Pain. Oxford University Press. | ||
| − | + | * Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., ''Prediction of Protein Structures and the Principles of Protein Conformation''. New York: Plenum Press. ISBN 0306431319. | |
| − | + | * International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. [http://www.chem.qmul.ac.uk/iupac/AminoAcid Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology]. ''IUPAC-IUB''. Retrieved June 14, 2007. | |
| + | * Kendall, E. C., and B. F. McKenzie. 1941. [http://www.orgsyn.org/orgsyn/pdfs/CV1P0021.pdf dl-Alanine]. ''Organic Syntheses'' 1: 21. | ||
| + | * Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. ''Lehninger Principles of Biochemistry'', 3rd ed. New York: Worth Publishing. ISBN 1572591536. | ||
| + | |||
{{AminoAcids}} | {{AminoAcids}} | ||
Revision as of 22:52, 29 June 2007
- For the eletronic brand see ProLine (company)
| Proline | |
|---|---|
| |
| 200px | |
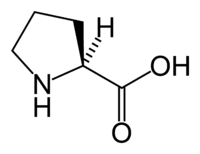
| IUPAC name | (S)-Pyrrolidine-2- carboxylic acid |
| Other names | Pro, P |
| Identifiers | |
| CAS number | [] |
| PubChem | |
| SMILES | OC(=O)[C@@H]1CCCN1 |
| Properties | |
| Molecular formula | C5H9NO2 |
| Molar mass | 115.13 g/mol |
| Melting point |
221 °C |
| Acidity (pKa) | 1.95, 10.47 |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) | |
L-Proline is one of the twenty proteinogenic units which are used in living organisms as the building blocks of proteins. The other nineteen units are all primary amino acids, but due to the cyclic binding of the three-carbon side chain to the nitrogen of the backbone, proline lacks a primary amine group (−NH2). The nitrogen in proline is properly referred to as a secondary amine. Proline is sometimes called an imino acid, although the IUPAC definition of an imine requires a carbon-nitrogen double bond. In biological terminology, however, the category "amino acids" is generally taken to include proline.
Serine is an α-amino acid that is common in many proteins, sometimes in substantial concentrations in the outer regions of soluble proteins due to its hydrophilic nature. Serine is an important component of phospholipids and participates in the biosynthesis of purines and pyriminidines, as well as such amino acids as cysteine and glycine. With an easily removed hydrogen on the hydroxyl side chain, serine is often a hydrogen donor in enzymes, such as trypsin and chymotrypsin, playing an important role in their function as catalysts.
In humans, the L-isomer, which is the only form that is involved in protein synthesis, is one of the 20 standard amino acids required for normal functioning. However, it is considered to be a "non-essential" amino acid since it does not have to be taken in with the diet, but can be synthesized by the human body from other compounds through chemical reactions.
Human creativity, which can be used for good or ill purposes, has exploited serine's role in the active site of the enzyme acetylcholine esterase to produce both nerve gases, such as Sarin that causes painful deaths in humans, and insecticides, which are designed to increase human agricultural productivity and prosperity. (See function below.)
Serine's three letter code is Ser, its one letter code is S, its codons are AGU and AGC, and its systematic name is 2-Amino-3-hydroxypropanoic acid (IUPAC-IUB 1983). The name serine was derived from the Latin for silk, "sericum," since serine was first isolated from silk protein. While the amino acids glycine and alanine make up the bulk of silk protein, it is also a rich source of serine.
Proline Pro P Pyrrolidine-2-carboxylic acid
Structure
In biochemistry, the term amino acid is frequently used to refer specifically to alpha amino acids: those amino acids in which the amino and carboxylate groups are attached to the same carbon, the so-called α–carbon (alpha carbon). The general structure of these alpha amino acids is:
R
|
H2N-C-COOH
|
H
where R represents a side chain specific to each amino acid. The exception to this basic structure is proline, whose side chain cyclizes onto the backbone, forming a ring structure in which a secondary amino group replaces the primary amino group.
Most amino acids occur in two possible optical isomers, called D and L. The L amino acids represent the vast majority of amino acids found in proteins. They are called proteinogenic amino acids. As the name "proteinogenic" (literally, protein building) suggests, these amino acid are encoded by the standard genetic code and participate in the process of protein synthesis. In serine, only the L-stereoisomer is involved in synthesis of mammalian proteins.
Serine has the chemical formula HO-CH2-CH(NH2)-COOH (alternatively, HO2C-CH(NH2)-CH2-OH), or more generally, C3H7NO3.
Serine, like threonine, has a short group ended with a hydroxyl group. The hydroxyl group attached makes it a polar amino acid. Its hydrogen is easy to remove, so serine and threonine often act as hydrogen donors in enzymes. Both are very hydrophilic, therefore the outer regions of soluble proteins tend to be rich with them.
The distinctive cyclic structure of proline's side chain locks its backbone dihedral angle at approximately -75°, giving proline an exceptional conformational rigidity compared to other amino acids. Hence, proline loses less conformational entropy upon folding, which may account for its higher prevalence in the proteins of thermophilic organisms.
Proline acts as a structural disruptor in the middle of regular secondary structure elements such as alpha helices and beta sheets; however, proline is commonly found as the first residue of an alpha helix and also in the edge strands of beta sheets. Proline is also commonly found in turns, which may account for the curious fact that proline is usually solvent-exposed, despite having a completely aliphatic side chain. Because proline lacks a hydrogen on the amide group, it cannot act as a hydrogen bond donor, only as a hydrogen bond acceptor.
Multiple prolines and/or hydroxyprolines in a row can create a polyproline helix, the predominant secondary structure in collagen. The hydroxylation of proline by prolyl hydroxylase (or other additions of electron-withdrawing substituents such as fluorine) increases the conformational stability of collagen significantly. Hence, the hydroxylation of proline is a critical biochemical process for maintaining the connective tissue of higher organisms. Severe diseases such as scurvy can result from defects in this hydroxylation, e.g., mutations in the enzyme prolyl hydroxylase or lack of the necessary ascorbate (vitamin C) cofactor.
Sequences of proline and 2-aminoisobutyric acid (Aib) also form a helical turn structure[citation needed].
Cis-trans isomerization
Peptide bonds to proline and other N-substituted amino acids (such as sarcosine) are able to populate both the cis and trans isomers. Most peptide bonds prefer overwhelmingly to adopt the trans isomer (typically 99.9% under unstrained conditions), chiefly because the amide hydrogen (trans isomer) offers less steric repulsion to the preceding atom than does the following atom (cis isomer). By contrast, the cis and trans isomers of the X-Pro peptide bond are nearly isosteric (i.e., equally bad energetically); the (cis isomer) and atoms (trans isomer) of proline are roughly equivalent sterically. Hence, the fraction of X-Pro peptide bonds in the cis isomer under unstrained conditions ranges from 10-40%; the fraction depends slightly on the preceding amino acid X, with aromatic residues favoring the cis isomer slightly.
Cis-trans proline isomerization is a very slow process that can impede the progress of protein folding by trapping one or more prolines crucial for folding in the nonnative isomer, especially when the native isomer is the rarer cis. All organisms possess prolyl isomerase enzymes to catalyze this isomerization, and some bacteria have specialized prolyl isomerases associated with the ribosome. However, not all prolines are essential for folding, and protein folding may proceed at a normal rate despite having non-native isomers of many X-Pro peptide bonds.
Synthesis and usage
Proline is biosynthetically derived from the amino acid L-glutamate and its direct precursor is the real imino acid (S)-Δ1-pyrroline-5-carboxylate (P5C).
Proline and its derivatives are often used as asymmetric catalysts in organic reactions. The CBS reduction or proline catalysed aldol condensation are prominent examples.
Proline has a sweet flavor with a distinct aftertaste. Proline also causes slight irritation to the tongue like Sichuan Pepper[citation needed].
For unknown reasons, L-proline is used as an ingredient in energy drinks such as "Sobe power fruit punch".
See also
- Collagen
- Polyproline helix
- Peptide bond (for more discussion of cis-trans isomerization)
- For a thorough scientific overview of disorders of proline and hydroxyproline metabolism, one can consult chapter 81 of OMMBID[1]. For more online resources and references, see inborn errors of metabolism.
External links
Template:ChemicalSources
ReferencesISBN links support NWE through referral fees
- Balbach J, Schmid FX. (2000). Proline isomerization and its catalysis in protein folding. In Mechanisms of Protein Folding 2nd ed. Editor RH Pain. Oxford University Press.
- Doolittle, R. F. 1989. Redundancies in protein sequences. In G. D. Fasman, ed., Prediction of Protein Structures and the Principles of Protein Conformation. New York: Plenum Press. ISBN 0306431319.
- International Union of Pure and Applied Chemistry and International Union of Biochemistry and Molecular Biology (IUPAC-IUB) Joint Commission on Biochemical Nomenclature. 1983. Nomenclature and symbolism for amino acids and peptides: Recommendations on organic & biochemical nomenclature, symbols & terminology. IUPAC-IUB. Retrieved June 14, 2007.
- Kendall, E. C., and B. F. McKenzie. 1941. dl-Alanine. Organic Syntheses 1: 21.
- Lehninger, A. L., D. L. Nelson, and M. M. Cox. 2000. Lehninger Principles of Biochemistry, 3rd ed. New York: Worth Publishing. ISBN 1572591536.
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.
- ↑ Charles Scriver, Beaudet, A.L., Valle, D., Sly, W.S., Vogelstein, B., Childs, B., Kinzler, K.W. (Accessed 2007). The Online Metabolic and Molecular Bases of Inherited Disease. New York: McGraw-Hill. - Summaries of 255 chapters, full text through many universities. There is also the OMMBID blog.


