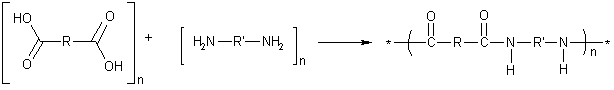Difference between revisions of "Nylon" - New World Encyclopedia
(→Chemistry: editing) |
|||
| Line 19: | Line 19: | ||
The name '''nylon''' is given to a family of [[synthetic polymer]]*s first produced on February 28, 1935, by Gerard J. Berchet of Wallace Carothers' research group at DuPont (E.I. du Pont de Nemours and Company) in Delaware. The first commercial product to use the material was a nylon-bristled toothbrush (in 1938), followed more famously by women's nylon stockings (in 1940). Nylon was the first commercially successful polymer and the first synthetic [[fiber]] to be made entirely from building blocks derived from [[coal]], in the presence of water and air. It was intended to be a synthetic replacement for [[silk]] and substituted for it in [[parachute]]s after the [[United States]] entered [[World War II]] in 1941, making stockings hard to find until the war's end. Nylon fibers are now used in [[fabric]]s and [[rope]]s, and solid nylon is used for [[machine|mechanical]] parts and as an [[engineering]] material. | The name '''nylon''' is given to a family of [[synthetic polymer]]*s first produced on February 28, 1935, by Gerard J. Berchet of Wallace Carothers' research group at DuPont (E.I. du Pont de Nemours and Company) in Delaware. The first commercial product to use the material was a nylon-bristled toothbrush (in 1938), followed more famously by women's nylon stockings (in 1940). Nylon was the first commercially successful polymer and the first synthetic [[fiber]] to be made entirely from building blocks derived from [[coal]], in the presence of water and air. It was intended to be a synthetic replacement for [[silk]] and substituted for it in [[parachute]]s after the [[United States]] entered [[World War II]] in 1941, making stockings hard to find until the war's end. Nylon fibers are now used in [[fabric]]s and [[rope]]s, and solid nylon is used for [[machine|mechanical]] parts and as an [[engineering]] material. | ||
| − | + | == Synthesis == | |
| − | |||
| − | |||
Nylons are composed of long-chain molecules, or ''polymers'', made by linking smaller building blocks, or ''monomers''. Most nylons are formed by reacting two types of building blocks—a ''diamine'' (which is a chemical base) and a ''dicarboxylic acid'' (which, as its name suggests, is an acid). Special types of bonds, called ''amide bonds'' (or ''peptide bonds''), link up these monomers into long chains. The polymer is therefore classified as a ''polyamide'' (PA). The generalized reaction can be written as follows. | Nylons are composed of long-chain molecules, or ''polymers'', made by linking smaller building blocks, or ''monomers''. Most nylons are formed by reacting two types of building blocks—a ''diamine'' (which is a chemical base) and a ''dicarboxylic acid'' (which, as its name suggests, is an acid). Special types of bonds, called ''amide bonds'' (or ''peptide bonds''), link up these monomers into long chains. The polymer is therefore classified as a ''polyamide'' (PA). The generalized reaction can be written as follows. | ||
| Line 29: | Line 27: | ||
This diagram indicates that "n" molecules of a dicarboxylic acid (on the left) react with "n" molecules of a diamine, producing a long chain in which the two monomers take up alternate positions and are repeated "n" times. As each amide bond is formed, a molecule of [[water]] is given off, and the reaction is therefore categorized as a ''condensation reaction''. The properties of the polymer are determined by the structures of the groups represented as R and R' in the monomers shown above. | This diagram indicates that "n" molecules of a dicarboxylic acid (on the left) react with "n" molecules of a diamine, producing a long chain in which the two monomers take up alternate positions and are repeated "n" times. As each amide bond is formed, a molecule of [[water]] is given off, and the reaction is therefore categorized as a ''condensation reaction''. The properties of the polymer are determined by the structures of the groups represented as R and R' in the monomers shown above. | ||
| − | The most common form of nylon is called Nylon 6,6, or Nylon 66, referring to the fact that the diamine (hexamethylene diamine) and the dicarboxylic acid (adipic acid) each contribute 6 [[carbon]] atoms to the polymer chain. (In the laboratory, Nylon 6,6 can also be made using [[adipoyl chloride]]* instead of adipic acid.) The numerical suffixes specify the number of carbon atoms donated by each monomer—the diamine first, the dicarboxylic acid, second | + | The most common form of nylon is called Nylon 6,6, or Nylon 66, referring to the fact that the diamine (hexamethylene diamine) and the dicarboxylic acid (adipic acid) each contribute 6 [[carbon]] atoms to the polymer chain. (In the laboratory, Nylon 6,6 can also be made using [[adipoyl chloride]]* instead of adipic acid.) The numerical suffixes specify the number of carbon atoms donated by each monomer—the diamine first, the dicarboxylic acid, second. |
In synthesizing nylon, it is difficult to get the diamine (base) and diacid in exactly 1:1 proportion, and the reaction may terminate before the polymer chains are sufficiently long. To overcome this problem, a [[crystal]]line, solid "nylon [[salt]]" can be formed at room temperature, using an exact 1:1 ratio of the acid and base to neutralize each other. In practice, especially for Nylon 6,6, the monomers are often combined in a water solution. The water used to make the solution is evaporated under controlled conditions, and the increasing concentration of "salt" is polymerized by heating, until the molecules reach the desired molecular weight. | In synthesizing nylon, it is difficult to get the diamine (base) and diacid in exactly 1:1 proportion, and the reaction may terminate before the polymer chains are sufficiently long. To overcome this problem, a [[crystal]]line, solid "nylon [[salt]]" can be formed at room temperature, using an exact 1:1 ratio of the acid and base to neutralize each other. In practice, especially for Nylon 6,6, the monomers are often combined in a water solution. The water used to make the solution is evaporated under controlled conditions, and the increasing concentration of "salt" is polymerized by heating, until the molecules reach the desired molecular weight. | ||
| + | |||
| + | == Varieties of nylons == | ||
DuPont patented<ref>[http://www.caimateriali.org/Eventi/Torino/historynylon.html History of Nylon] US Patent 2,130,523 'Linear polyamides suitable for spinning into strong pliable fibers', U.S. Patent 2,130,947 'Diamine dicarboxylic acid salt' issued and U.S. Patent 2,130,948 'Synthetic fibers', all issued 20 September 1938</ref> Nylon 6,6. So in order to compete, other companies (particularly the German BASF) developed Nylon 6, in which each chain is made from a single type of monomer called ''caprolactam''. The properties of Nylon 6 are sometimes indistinguishable from those of Nylon 6,6—except for the melting temperature (N6 is lower) and some fiber properties in products like carpets and textiles. Another product that has been developed is called Nylon 9. | DuPont patented<ref>[http://www.caimateriali.org/Eventi/Torino/historynylon.html History of Nylon] US Patent 2,130,523 'Linear polyamides suitable for spinning into strong pliable fibers', U.S. Patent 2,130,947 'Diamine dicarboxylic acid salt' issued and U.S. Patent 2,130,948 'Synthetic fibers', all issued 20 September 1938</ref> Nylon 6,6. So in order to compete, other companies (particularly the German BASF) developed Nylon 6, in which each chain is made from a single type of monomer called ''caprolactam''. The properties of Nylon 6 are sometimes indistinguishable from those of Nylon 6,6—except for the melting temperature (N6 is lower) and some fiber properties in products like carpets and textiles. Another product that has been developed is called Nylon 9. | ||
| Line 44: | Line 44: | ||
* Engineering grade Nylon is processed by extrusion, casting & injection molding. Type 6/6 Nylon 101 is the most common commercial grade of Nylon, and Nylon 6 is the most common commercial grade of cast Nylon. | * Engineering grade Nylon is processed by extrusion, casting & injection molding. Type 6/6 Nylon 101 is the most common commercial grade of Nylon, and Nylon 6 is the most common commercial grade of cast Nylon. | ||
| − | * a [[thermoplastic]] material | + | ==Bulk properties== |
| + | |||
| + | ** a [[thermoplastic]] material | ||
| − | |||
Above their [[glass transition temperature|melting temperatures]], ''T''<sub>m</sub>, [[thermoplastic]]s like nylon are [[amorphous solid]]s or viscous [[fluid]]s in which the chains approximate [[random coil]]s. Below ''T''<sub>m</sub>, amorphous regions alternate with regions which are [[lamellae (materials)|lamellar]] [[crystal]]s.[http://aml.arizona.edu/classes/mse222/1998/nylon66/mse222.htm] The amorphous regions contribute elasticity and the crystalline regions contribute strength and rigidity. The [[planar]] amide (-CO-NH-) groups are very [[chemical polarity|polar]], so nylon forms multiple [[hydrogen bond]]s among adjacent strands. Because the nylon backbone is so regular and symmetrical, especially if all the amide bonds are in the [[geometric isomerism|''trans'' configuration]], nylons often have high crystallinity and make excellent fibers. The amount of crystallinity depends on the details of formation, as well as on the kind of nylon. Apparently it can never be [[quench]]ed from a [[melt]] as a completely amorphous solid. | Above their [[glass transition temperature|melting temperatures]], ''T''<sub>m</sub>, [[thermoplastic]]s like nylon are [[amorphous solid]]s or viscous [[fluid]]s in which the chains approximate [[random coil]]s. Below ''T''<sub>m</sub>, amorphous regions alternate with regions which are [[lamellae (materials)|lamellar]] [[crystal]]s.[http://aml.arizona.edu/classes/mse222/1998/nylon66/mse222.htm] The amorphous regions contribute elasticity and the crystalline regions contribute strength and rigidity. The [[planar]] amide (-CO-NH-) groups are very [[chemical polarity|polar]], so nylon forms multiple [[hydrogen bond]]s among adjacent strands. Because the nylon backbone is so regular and symmetrical, especially if all the amide bonds are in the [[geometric isomerism|''trans'' configuration]], nylons often have high crystallinity and make excellent fibers. The amount of crystallinity depends on the details of formation, as well as on the kind of nylon. Apparently it can never be [[quench]]ed from a [[melt]] as a completely amorphous solid. | ||
Revision as of 17:04, 24 July 2006
- For other uses of this word, see nylon (disambiguation).
 Nylon Nylon
| |
|---|---|
| Density | 1.15 g/cm³ |
| Electrical conductivity (σ) | 10-12 S/m |
| Thermal conductivity | 0.25 W/(m·K) |
| Melting points | 463 K-624 K 190°C-350°C 374°F-663°F |
The name nylon is given to a family of synthetic polymers first produced on February 28, 1935, by Gerard J. Berchet of Wallace Carothers' research group at DuPont (E.I. du Pont de Nemours and Company) in Delaware. The first commercial product to use the material was a nylon-bristled toothbrush (in 1938), followed more famously by women's nylon stockings (in 1940). Nylon was the first commercially successful polymer and the first synthetic fiber to be made entirely from building blocks derived from coal, in the presence of water and air. It was intended to be a synthetic replacement for silk and substituted for it in parachutes after the United States entered World War II in 1941, making stockings hard to find until the war's end. Nylon fibers are now used in fabrics and ropes, and solid nylon is used for mechanical parts and as an engineering material.
Synthesis
Nylons are composed of long-chain molecules, or polymers, made by linking smaller building blocks, or monomers. Most nylons are formed by reacting two types of building blocks—a diamine (which is a chemical base) and a dicarboxylic acid (which, as its name suggests, is an acid). Special types of bonds, called amide bonds (or peptide bonds), link up these monomers into long chains. The polymer is therefore classified as a polyamide (PA). The generalized reaction can be written as follows.
This diagram indicates that "n" molecules of a dicarboxylic acid (on the left) react with "n" molecules of a diamine, producing a long chain in which the two monomers take up alternate positions and are repeated "n" times. As each amide bond is formed, a molecule of water is given off, and the reaction is therefore categorized as a condensation reaction. The properties of the polymer are determined by the structures of the groups represented as R and R' in the monomers shown above.
The most common form of nylon is called Nylon 6,6, or Nylon 66, referring to the fact that the diamine (hexamethylene diamine) and the dicarboxylic acid (adipic acid) each contribute 6 carbon atoms to the polymer chain. (In the laboratory, Nylon 6,6 can also be made using adipoyl chloride instead of adipic acid.) The numerical suffixes specify the number of carbon atoms donated by each monomer—the diamine first, the dicarboxylic acid, second.
In synthesizing nylon, it is difficult to get the diamine (base) and diacid in exactly 1:1 proportion, and the reaction may terminate before the polymer chains are sufficiently long. To overcome this problem, a crystalline, solid "nylon salt" can be formed at room temperature, using an exact 1:1 ratio of the acid and base to neutralize each other. In practice, especially for Nylon 6,6, the monomers are often combined in a water solution. The water used to make the solution is evaporated under controlled conditions, and the increasing concentration of "salt" is polymerized by heating, until the molecules reach the desired molecular weight.
Varieties of nylons
DuPont patented[1] Nylon 6,6. So in order to compete, other companies (particularly the German BASF) developed Nylon 6, in which each chain is made from a single type of monomer called caprolactam. The properties of Nylon 6 are sometimes indistinguishable from those of Nylon 6,6—except for the melting temperature (N6 is lower) and some fiber properties in products like carpets and textiles. Another product that has been developed is called Nylon 9.
A wide range of other nylons have been produced and are named using the above-mentioned convention. For instance, "Nylon 6,12" (N-6,12) or "PA-6,12" is a copolymer of a 6-carbon diamine and a 12-carbon diacid. Likewise, nylons named N-5,10, N-6,11, and N-10,12 have been made.
Additional varieties of nylons include copolymerized dicarboxylic acid/diamine products that are not based upon the monomers listed above. For example, some "aromatic" nylons are polymerized with the addition of diacids like terephthalic acid to produce Kevlar, or isophthalic acid to produce Nomex. There are also copolymers of N-6,6/N6; copolymers of N-6,6/N-6/N-12; and others.
Given the way polyamides are formed, nylon would seem to be limited to unbranched, straight chains. Yet "star" branched nylon can be produced by the condensation of dicarboxylic acids with polyamines having three or more amino (NH2) groups.
- Engineering grade Nylon is processed by extrusion, casting & injection molding. Type 6/6 Nylon 101 is the most common commercial grade of Nylon, and Nylon 6 is the most common commercial grade of cast Nylon.
Bulk properties
- a thermoplastic material
Above their melting temperatures, Tm, thermoplastics like nylon are amorphous solids or viscous fluids in which the chains approximate random coils. Below Tm, amorphous regions alternate with regions which are lamellar crystals.[1] The amorphous regions contribute elasticity and the crystalline regions contribute strength and rigidity. The planar amide (-CO-NH-) groups are very polar, so nylon forms multiple hydrogen bonds among adjacent strands. Because the nylon backbone is so regular and symmetrical, especially if all the amide bonds are in the trans configuration, nylons often have high crystallinity and make excellent fibers. The amount of crystallinity depends on the details of formation, as well as on the kind of nylon. Apparently it can never be quenched from a melt as a completely amorphous solid.
Nylon 6,6 can have multiple parallel strands aligned with their neighboring peptide bonds at coordinated separations of exactly 6 and 4 carbons for considerable lengths, so the carbonyl oxygens and amide hydrogens can line up to form interchain hydrogen bonds repeatedly, without interruption. Nylon 5,10 can have coordinated runs of 5 and 8 carbons. Thus parallel (but not antiparallel) strands can participate in extended, unbroken, multi-chain β-pleated sheets, a strong and tough supermolecular structure similar to that found in natural silk fibroin and the β-keratins in feathers. (Proteins have only an amino acid α-carbon separating sequential -CO-NH- groups.) Nylon 6 will form uninterrupted H-bonded sheets with mixed directionalities, but the β-sheet wrinkling is somewhat different. The three-dimensional disposition of each alkane hydrocarbon chain depends on rotations about the 109.47° tetrahedral bonds of singly-bonded carbon atoms.
When extruded into fibers through pores in an industrial spinneret, the individual polymer chains tend to align because of viscous flow. If subjected to cold drawing afterwards, the fibers align further, increasing their crystallinity, and the material acquires additional tensile strength.[2] Block nylon tends to be less crystalline, except near the surfaces due to shearing stresses during formation. Nylon is clear and colorless, or milky, but is easily dyed. Multistranded nylon cord and rope is slippery and tends to unravel. The ends can be melted and fused with a flame to prevent this.
There are carbon fiber/nylon composities with higher density than pure nylon.
Historical uses
During World War II, nylon replaced Asian silk in parachutes. It was also used to make tires, tents, ropes, ponchos, and other military supplies. It was even used in the production of a high-grade paper for U.S. currency. At the outset of the war, cotton accounted for more than 80% of all fibers used, and manufactured and wool fibers accounted for the remaining 20%. By August, 1945, manufactured fibers had taken a market share of 25% and cotton had dropped.
Some people, such as Jack Herer, surmise that Cannabis sativa was made illegal because the fibers from the hemp plant, used for fabrics and ropes, were in strong competition with nylon (along with paper, fuel, and other industries). While the production of rope from hemp requires no chemicals or industrial processes, nylon fiber is more than twice as strong as hemp and weighs 25% less. An additional problem is that hemp rope rots from the inside out, making it difficult to determine the condition of a rope at a glance. While hemp was originally used in climbing rope, this is no longer the case, even in countries where cannabis is legal.
Some of the terpolymers based upon nylon are used every day in packaging. Nylon has been used for meat wrappings and sausage sheaths.
Etymology
In 1940 John W. Eckelberry of DuPont stated that the letters "nyl" were arbitrary and the "on" was copied from the names of other fibers such as cotton and rayon. A later publication by DuPont (Context, vol. 7, no. 2, 1978) explained that the name was originally intended to be "No-Run" ("run" meaning "unravel"), but was modified to avoid making such an unjustified claim and to make the word sound better. The story goes that Carothers changed one letter at a time until DuPont's management was satisfied. But he was not involved in the nylon project during the last year of his life, and committed suicide before the name was coined. There is another story (repeated in James Burke's TV series Connections) that another one of the names considered was to be Duparooh for DUpont Pulls A Rabbit Out Of a Hat. Nylon was never trademarked. Another popular myth is that "Nylon" stands for "Now You Lousy Old Nippons". Yet another explanation is that it stands for "New York-London", the source of the chemists working on the materials sythesis, but there is no evidence that nylon was named after New York and London.
Uses
- nylon fiber
- clothing
- pantyhose
- toothbrush bristles
- fishing lines
- nets
- carpet fiber
- airbag fiber
- auto parts: intake manifolds, gas (petrol) tanks
- slings and rope used in climbing gear
- machine parts, such as gears and bearings
- parachutes
- metallized nylon balloons
- classical and flamenco guitar strings
- paintball marker bolts
- racquetball, squash, and tennis racquet strings
See also
Footnotes
- ↑ History of Nylon US Patent 2,130,523 'Linear polyamides suitable for spinning into strong pliable fibers', U.S. Patent 2,130,947 'Diamine dicarboxylic acid salt' issued and U.S. Patent 2,130,948 'Synthetic fibers', all issued 20 September 1938
External links
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.