Difference between revisions of "Fluorine" - New World Encyclopedia
Rosie Tanabe (talk | contribs) |
|||
| (24 intermediate revisions by 6 users not shown) | |||
| Line 1: | Line 1: | ||
| + | {{Copyedited}}{{Paid}}{{Images OK}}{{Submitted}}{{Approved}} | ||
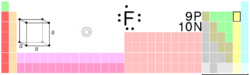
{{Elementbox_header | number=9 | symbol=F | name=fluorine | left=[[oxygen]] | right=[[neon]] | above=- | below=[[chlorine|Cl]] | color1=#ffff99 | color2=green }} | {{Elementbox_header | number=9 | symbol=F | name=fluorine | left=[[oxygen]] | right=[[neon]] | above=- | below=[[chlorine|Cl]] | color1=#ffff99 | color2=green }} | ||
{{Elementbox_series | [[halogen]]s }} | {{Elementbox_series | [[halogen]]s }} | ||
| Line 21: | Line 22: | ||
{{Elementbox_electroneg_pauling | 3.98 }} | {{Elementbox_electroneg_pauling | 3.98 }} | ||
{{Elementbox_ionizationenergies4 | 1681.0 | 3374.2 | 6050.4 }} | {{Elementbox_ionizationenergies4 | 1681.0 | 3374.2 | 6050.4 }} | ||
| − | {{Elementbox_atomicradius_pm | + | {{Elementbox_atomicradius_pm | 50 }} |
| − | {{Elementbox_atomicradiuscalc_pm | + | {{Elementbox_atomicradiuscalc_pm | 42 }} |
| − | {{Elementbox_covalentradius_pm | + | {{Elementbox_covalentradius_pm | 71 }} |
| − | {{Elementbox_vanderwaalsrad_pm | + | {{Elementbox_vanderwaalsrad_pm | 147 }} |
{{Elementbox_section_miscellaneous | color1=#ffff99 | color2=green }} | {{Elementbox_section_miscellaneous | color1=#ffff99 | color2=green }} | ||
{{Elementbox_magnetic | nonmagnetic }} | {{Elementbox_magnetic | nonmagnetic }} | ||
| Line 34: | Line 35: | ||
{{Elementbox_footer | color1=#ffff99 | color2=green }} | {{Elementbox_footer | color1=#ffff99 | color2=green }} | ||
| − | '''Fluorine''' (chemical symbol '''F''', [[atomic number]] 9) is a nonmetal that belongs to a | + | '''Fluorine''' (chemical symbol '''F''', [[atomic number]] 9) is a nonmetal that belongs to a group of [[chemical element]]s known as ''halogens''. Chemically, it is the most reactive and [[Electronegativity|electronegative]] of all elements. At ordinary temperatures and pressures, pure fluorine is a [[poison]]ous [[gas]], pale yellow in color, with the chemical formula F<sub>2</sub>. Like other [[halogen]]s, molecular fluorine is extremely dangerous, causing severe chemical burns on contact with skin. |
| + | {{toc}} | ||
| + | Fluorine and its compounds are useful for a wide range of applications, including the manufacture of pharmaceuticals, agrochemicals, lubricants, and textiles. [[Hydrofluoric acid]] is used to etch [[glass]], and fluorine is used for [[plasma etching]] in the manufacture of [[semiconductor]]s and other products. Low concentrations of fluoride ions in toothpaste and drinking water can help prevent dental cavities, while higher concentrations of fluoride are used in some insecticides. Many important general anesthetics are derivatives of [[fluorohydrocarbon]]s. The isotope <sup>18</sup>F is a source of [[positron]]s for medical imaging by the technique called PET ([[positron emission tomography]]), and uranium hexafluoride is used to separate [[uranium]] isotopes and produce enriched uranium for [[nuclear power]] plants. | ||
| + | == Discovery and isolation == | ||
| − | + | The name fluorine is derived from the [[Latin]] term ''fluere'', meaning "to flow." Minerals containing compounds of fluorine were known for many years before isolation of the element fluorine. For example, the mineral fluorspar (or fluorite), consisting of calcium fluoride, was described in 1530 by [[Georg Agricola|Georgius Agricola]].<ref>Peter Meiers, [http://www.fluoride-history.de/fluorine.htm “Discovery of fluorine,”] Fluoride-History.de. Retrieved September 7, 2007.</ref> He noted that it was useful as a [[Flux (metallurgy)|flux]]—a substance that helps lower the melting temperature of a [[metal]] or [[ore]] and aids in purification of the desired metal. | |
| − | |||
| − | |||
| − | + | In 1670, the glassworker Heinrich Schwanhard found that glass was etched when exposed to [[acid]]-treated fluorspar. [[Karl Scheele]] and many later researchers—including [[Humphry Davy]], [[Joseph Louis Gay-Lussac]], [[Antoine Lavoisier]], and [[Louis Jacques Thenard|Louis Thenard]]—experimented with hydrofluoric acid (HF), which was readily obtained by treating calcium fluoride (fluorspar) with concentrated sulfuric acid. | |
| − | + | It was eventually realized that hydrofluoric acid contained a previously unknown element. This element, however, was not isolated for many years, because of its extreme reactivity. Not only is it difficult to separate from its compounds, but it then immediately attacks the remaining materials of the compound. The derivation of elemental fluorine from hydrofluoric acid is exceptionally dangerous, and early attempts to do so blinded and killed several scientists. These men came to be known as the "fluorine martyrs." | |
| − | + | Finally, French chemist [[Henri Moissan]] succeeded in isolating fluorine in 1886, through the [[electrolysis]] of a mixture of molten potassium fluoride and hydrofluoric acid. For that success, Moissan was awarded the 1906 [[Nobel Prize#Nobel Prize in Chemistry|Nobel Prize in Chemistry]]. His electrolytic approach continues to be used today for the industrial preparation of fluorine. | |
| − | + | The first large-scale production of fluorine was undertaken during [[World War II]], as a step in the making of [[atomic bomb]]s in the [[Manhattan project]]. The fluorine was used to produce [[uranium hexafluoride]] (UF<sub>6</sub>), which in turn was used to separate two uranium isotopes, <sup>235</sup>U and <sup>238</sup>U, from each other. Today, gaseous UF<sub>6</sub> is used to produce enriched [[uranium]] for [[nuclear power]] applications. | |
| − | + | == Notable characteristics == | |
| − | + | In the [[periodic table]], fluorine is located at the top of group 17 (former group 7A), which is the halogen family. Other halogens are [[chlorine]], [[bromine]], [[iodine]], and [[astatine]]. In addition, it is situated in period 2, between [[oxygen]] and [[neon]]. | |
| − | + | Pure fluorine (chemical formula F<sub>2</sub>) is a corrosive [[gas]] with a characteristic pungent odor that is detectable in concentrations as low as 20 nanoliters per liter of gas volume. As the most reactive and electronegative of all elements, it readily forms compounds with most other elements. It is far too reactive to be found in elemental form and has such an affinity for most elements, including [[silicon]], that it cannot be prepared or stored in glass vessels. In moist air, it reacts with water to form the equally dangerous [[hydrofluoric acid]]. | |
| − | + | Fluorine reacts explosively with [[hydrogen]] even under cool conditions in the dark. It reacts violently with [[water]] to generate hydrofluoric acid and [[oxygen]] gas. Various materials—including finely divided [[metal]]s and [[glass]]—burn with a bright flame in a jet of fluorine gas. Moreover, fluorine forms compounds with the [[noble gas]]es [[krypton]], [[xenon]], and [[radon]]. It does not, however, react directly with [[nitrogen]] or oxygen. | |
| − | + | Notwithstanding its extreme reactivity, methods for the safe handling and transport of fluorine are now available. The element can be stored in containers of [[steel]] or Monel metal (a nickel-rich alloy of metals), as these materials form surface fluorides that resist further reaction. | |
| − | [[Fluoride]]s are compounds | + | [[Fluoride]]s are compounds in which fluorine occurs in the form of the negatively charged fluoride ion (F<sup>−</sup>), combined with some positively charged counterpart. Compounds of fluorine with metals are among the most stable of salts. When dissolved in water, these salts release fluoride ions. Other forms of fluorine are fluoro-[[complex (chemistry)|complex]]es, such as [FeF<sub>4</sub>]<sup>−</sup>, and H<sub>2</sub>F<sup>+</sup>. |
| − | == | + | === Isotopes === |
| − | |||
| − | + | There are many isotopes of fluorine, ranging from <sup>14</sup>F to <sup>31</sup>F. Only one of these isotopes, <sup>19</sup>F, which contains 10 neutrons, is stable. The radioactive isotope <sup>18</sup>F is a valuable source of [[positron]]s. | |
| − | + | == Biological effects == | |
| − | + | Bones and teeth contain most of the body's fluorine, in the form of fluoride ions. Fluoridation of drinking water (at levels below one part per million) significantly reduces the incidence of dental caries—a viewpoint endorsed by the Food and Nutrition Board of the National Academy of Sciences-National Research Council (NAS/NRC). On the other hand, excess accumulation of fluoride can lead to fluorosis, manifested in the mottling of teeth. This effect is commonly observed in communities with drinking water containing fluoride at concentrations exceeding ten parts per million. | |
| − | |||
| − | + | Both elemental fluorine and fluoride salts are toxic and must be handled with great care. Contact with the [[skin]] or [[eye]]s should be strictly avoided. Contact with exposed skin produces hydrofluoric acid, which rapidly migrates through the skin and flesh and reacts with calcium in the bones, permanently damaging the bones. | |
== Compounds == | == Compounds == | ||
| − | + | A wide range of organic and inorganic compounds contain fluorine. In the case of organic compounds, chemists can replace [[hydrogen]] atoms with fluorine atoms, thus creating many new products. Being a highly reactive element, fluorine forms compounds with several noble gases, as demonstrated by [[Neil Bartlett]] who synthesized xenon hexafluoroplatinate (XePtF<sub>6</sub>) in 1962. Fluorides of [[krypton]] and [[radon]] have also been prepared. Another compound is argon fluorohydride, but it is stable only at extremely low temperatures. | |
| − | [[Image:Fluorite_crystals_270x444.jpg|thumb|right|300px| | + | [[Image:Fluorite_crystals_270x444.jpg|thumb|right|300px|Crystals of the mineral fluorite (calcium fluoride, CaF<sub>2</sub>)]] |
| − | + | The following is a list of inorganic compounds of fluorine. | |
*[[Ammonium fluoride]] (NH<sub>4</sub>F) | *[[Ammonium fluoride]] (NH<sub>4</sub>F) | ||
*[[Antimony pentafluoride]] (SbF<sub>5</sub>) | *[[Antimony pentafluoride]] (SbF<sub>5</sub>) | ||
| Line 85: | Line 85: | ||
*[[Bromine pentafluoride]] (BrF<sub>5</sub>) | *[[Bromine pentafluoride]] (BrF<sub>5</sub>) | ||
*[[Bromine trifluoride]] (BrF<sub>3</sub>) | *[[Bromine trifluoride]] (BrF<sub>3</sub>) | ||
| − | *[[ | + | *[[Cesium fluoride]] (CsF) |
*[[Calcium fluoride]] (CaF<sub>2</sub>) | *[[Calcium fluoride]] (CaF<sub>2</sub>) | ||
*[[Chlorine pentafluoride]] (ClF<sub>5</sub>) | *[[Chlorine pentafluoride]] (ClF<sub>5</sub>) | ||
| − | *[[Fluorosulfuric acid]] (FSO<sub>3</sub> | + | *[[Fluorosulfuric acid]] (FSO<sub>3</sub>H) |
| − | *[[Hydrofluoric acid]] (HF) | + | *[[Hydrofluoric acid]] (HF) |
| − | *[[Iodine pentafluoride]] (IF<sub>5</sub>) | + | *[[Iodine pentafluoride]] (IF<sub>5</sub>) |
*[[Iodine heptafluoride]] (IF<sub>7</sub>) | *[[Iodine heptafluoride]] (IF<sub>7</sub>) | ||
*[[Lithium fluoride]] (LiF) | *[[Lithium fluoride]] (LiF) | ||
| Line 113: | Line 113: | ||
== Applications == | == Applications == | ||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | * Fluorine in the atomic and molecular states is used for [[plasma etching]] in the manufacture of [[semiconductor]]s, [[flat panel display]]s, and [[MEMS]] (microelectromechanical systems). | ||
| + | * [[Hydrofluoric acid]] is used to etch glass in light bulbs and other products. | ||
| + | * Along with some of its compounds, fluorine is useful for the manufacture of pharmaceuticals, agrochemicals, lubricants, and textiles. | ||
| + | * Fluorine is used indirectly in the production of low-friction [[plastic]]s such as [[Teflon]]. | ||
| + | * Fluorine has been used in producing halogenated [[alkane]]s ([[halon]]s), which in turn have been used extensively in [[air conditioning]] and [[refrigeration]]. [[Chlorofluorocarbon]]s (CFCs) have been banned for these applications because they contribute to expansion of the [[ozone]] hole in the upper atmosphere. | ||
| + | * [[Sulfur hexafluoride]] is an extremely inert, nontoxic gas, and a member of a class of compounds that are potent [[greenhouse gas]]es. | ||
| + | * Many important agents for general anesthesia (such as [[sevoflurane]], [[desflurane]], and [[isoflurane]]) are derivatives of [[fluorohydrocarbon]]s. | ||
| + | * [[Sodium]] hexafluoroaluminate (cryolite) is used in the electrolysis of aluminum. | ||
| + | * Compounds of fluorine, including [[sodium fluoride]], are used in [[toothpaste]] to prevent dental cavities. These compounds are also added to municipal water supplies, a process called [[water fluoridation]], but health concerns have led to controversy over this practice. | ||
| + | * At much higher concentrations, [[sodium fluoride]] has been used as an insecticide, especially against cockroaches. | ||
| + | * Fluorides have been used in the past to reduce the melting temperatures of metals and ores, and to help them flow. | ||
| + | * [[Fluorine-18|<sup>18</sup>F]], a radioactive isotope of fluorine with a half-life of 110 minutes, emits [[positron]]s and is often used in medical imaging by a technique known as [[positron emission tomography]]. | ||
| + | * Fluorine is used for the production of [[uranium hexafluoride]], which in turn is used to separate uranium isotopes, as noted above. | ||
==See also== | ==See also== | ||
| − | * [[ | + | * [[Chemical element]] |
| − | * [[ | + | * [[Periodic table]] |
| − | * [[ | + | * [[Periodic table, main group elements]] |
| − | |||
| − | == | + | == Notes == |
<references/> | <references/> | ||
==References== | ==References== | ||
| − | |||
== External links == | == External links == | ||
| + | All links retrieved March 28, 2024. | ||
* [http://www.webelements.com/webelements/elements/text/F/index.html WebElements.com – Fluorine] | * [http://www.webelements.com/webelements/elements/text/F/index.html WebElements.com – Fluorine] | ||
* [http://education.jlab.org/itselemental/ele009.html It's Elemental – Fluorine] | * [http://education.jlab.org/itselemental/ele009.html It's Elemental – Fluorine] | ||
| − | |||
| − | |||
| − | |||
| − | |||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
[[Category:Chemistry]] | [[Category:Chemistry]] | ||
| − | + | ||
{{credit|73307931}} | {{credit|73307931}} | ||
Latest revision as of 17:47, 28 March 2024
| |||||||||||||||
| General | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Name, Symbol, Number | fluorine, F, 9 | ||||||||||||||
| Chemical series | halogens | ||||||||||||||
| Group, Period, Block | 17, 2, p | ||||||||||||||
| Appearance | Yellowish brown gas 
| ||||||||||||||
| Atomic mass | 18.9984032(5) g/mol | ||||||||||||||
| Electron configuration | 1s2 2s2 2p5 | ||||||||||||||
| Electrons per shell | 2, 7 | ||||||||||||||
| Physical properties | |||||||||||||||
| Phase | gas | ||||||||||||||
| Density | (0 °C, 101.325 kPa) 1.7 g/L | ||||||||||||||
| Melting point | 53.53 K (-219.62 °C, -363.32 °F) | ||||||||||||||
| Boiling point | 85.03 K (-188.12 °C, -306.62 °F) | ||||||||||||||
| Critical point | 144.13 K, 5.172 MPa | ||||||||||||||
| Heat of fusion | (F2) 0.510 kJ/mol | ||||||||||||||
| Heat of vaporization | (F2) 6.62 kJ/mol | ||||||||||||||
| Heat capacity | (25 °C) (F2) 31.304 J/(mol·K) | ||||||||||||||
| |||||||||||||||
| Atomic properties | |||||||||||||||
| Crystal structure | cubic | ||||||||||||||
| Oxidation states | −1 (strongly acidic oxide) | ||||||||||||||
| Electronegativity | 3.98 (Pauling scale) | ||||||||||||||
| Ionization energies (more) |
1st: 1681.0 kJ/mol | ||||||||||||||
| 2nd: 3374.2 kJ/mol | |||||||||||||||
| 3rd: 6050.4 kJ/mol | |||||||||||||||
| Atomic radius | 50 pm | ||||||||||||||
| Atomic radius (calc.) | 42 pm | ||||||||||||||
| Covalent radius | 71 pm | ||||||||||||||
| Van der Waals radius | 147 pm | ||||||||||||||
| Miscellaneous | |||||||||||||||
| Magnetic ordering | nonmagnetic | ||||||||||||||
| Thermal conductivity | (300 K) 27.7 mW/(m·K) | ||||||||||||||
| CAS registry number | 7782-41-4 | ||||||||||||||
| Notable isotopes | |||||||||||||||
| |||||||||||||||
Fluorine (chemical symbol F, atomic number 9) is a nonmetal that belongs to a group of chemical elements known as halogens. Chemically, it is the most reactive and electronegative of all elements. At ordinary temperatures and pressures, pure fluorine is a poisonous gas, pale yellow in color, with the chemical formula F2. Like other halogens, molecular fluorine is extremely dangerous, causing severe chemical burns on contact with skin.
Fluorine and its compounds are useful for a wide range of applications, including the manufacture of pharmaceuticals, agrochemicals, lubricants, and textiles. Hydrofluoric acid is used to etch glass, and fluorine is used for plasma etching in the manufacture of semiconductors and other products. Low concentrations of fluoride ions in toothpaste and drinking water can help prevent dental cavities, while higher concentrations of fluoride are used in some insecticides. Many important general anesthetics are derivatives of fluorohydrocarbons. The isotope 18F is a source of positrons for medical imaging by the technique called PET (positron emission tomography), and uranium hexafluoride is used to separate uranium isotopes and produce enriched uranium for nuclear power plants.
Discovery and isolation
The name fluorine is derived from the Latin term fluere, meaning "to flow." Minerals containing compounds of fluorine were known for many years before isolation of the element fluorine. For example, the mineral fluorspar (or fluorite), consisting of calcium fluoride, was described in 1530 by Georgius Agricola.[1] He noted that it was useful as a flux—a substance that helps lower the melting temperature of a metal or ore and aids in purification of the desired metal.
In 1670, the glassworker Heinrich Schwanhard found that glass was etched when exposed to acid-treated fluorspar. Karl Scheele and many later researchers—including Humphry Davy, Joseph Louis Gay-Lussac, Antoine Lavoisier, and Louis Thenard—experimented with hydrofluoric acid (HF), which was readily obtained by treating calcium fluoride (fluorspar) with concentrated sulfuric acid.
It was eventually realized that hydrofluoric acid contained a previously unknown element. This element, however, was not isolated for many years, because of its extreme reactivity. Not only is it difficult to separate from its compounds, but it then immediately attacks the remaining materials of the compound. The derivation of elemental fluorine from hydrofluoric acid is exceptionally dangerous, and early attempts to do so blinded and killed several scientists. These men came to be known as the "fluorine martyrs."
Finally, French chemist Henri Moissan succeeded in isolating fluorine in 1886, through the electrolysis of a mixture of molten potassium fluoride and hydrofluoric acid. For that success, Moissan was awarded the 1906 Nobel Prize in Chemistry. His electrolytic approach continues to be used today for the industrial preparation of fluorine.
The first large-scale production of fluorine was undertaken during World War II, as a step in the making of atomic bombs in the Manhattan project. The fluorine was used to produce uranium hexafluoride (UF6), which in turn was used to separate two uranium isotopes, 235U and 238U, from each other. Today, gaseous UF6 is used to produce enriched uranium for nuclear power applications.
Notable characteristics
In the periodic table, fluorine is located at the top of group 17 (former group 7A), which is the halogen family. Other halogens are chlorine, bromine, iodine, and astatine. In addition, it is situated in period 2, between oxygen and neon.
Pure fluorine (chemical formula F2) is a corrosive gas with a characteristic pungent odor that is detectable in concentrations as low as 20 nanoliters per liter of gas volume. As the most reactive and electronegative of all elements, it readily forms compounds with most other elements. It is far too reactive to be found in elemental form and has such an affinity for most elements, including silicon, that it cannot be prepared or stored in glass vessels. In moist air, it reacts with water to form the equally dangerous hydrofluoric acid.
Fluorine reacts explosively with hydrogen even under cool conditions in the dark. It reacts violently with water to generate hydrofluoric acid and oxygen gas. Various materials—including finely divided metals and glass—burn with a bright flame in a jet of fluorine gas. Moreover, fluorine forms compounds with the noble gases krypton, xenon, and radon. It does not, however, react directly with nitrogen or oxygen.
Notwithstanding its extreme reactivity, methods for the safe handling and transport of fluorine are now available. The element can be stored in containers of steel or Monel metal (a nickel-rich alloy of metals), as these materials form surface fluorides that resist further reaction.
Fluorides are compounds in which fluorine occurs in the form of the negatively charged fluoride ion (F−), combined with some positively charged counterpart. Compounds of fluorine with metals are among the most stable of salts. When dissolved in water, these salts release fluoride ions. Other forms of fluorine are fluoro-complexes, such as [FeF4]−, and H2F+.
Isotopes
There are many isotopes of fluorine, ranging from 14F to 31F. Only one of these isotopes, 19F, which contains 10 neutrons, is stable. The radioactive isotope 18F is a valuable source of positrons.
Biological effects
Bones and teeth contain most of the body's fluorine, in the form of fluoride ions. Fluoridation of drinking water (at levels below one part per million) significantly reduces the incidence of dental caries—a viewpoint endorsed by the Food and Nutrition Board of the National Academy of Sciences-National Research Council (NAS/NRC). On the other hand, excess accumulation of fluoride can lead to fluorosis, manifested in the mottling of teeth. This effect is commonly observed in communities with drinking water containing fluoride at concentrations exceeding ten parts per million.
Both elemental fluorine and fluoride salts are toxic and must be handled with great care. Contact with the skin or eyes should be strictly avoided. Contact with exposed skin produces hydrofluoric acid, which rapidly migrates through the skin and flesh and reacts with calcium in the bones, permanently damaging the bones.
Compounds
A wide range of organic and inorganic compounds contain fluorine. In the case of organic compounds, chemists can replace hydrogen atoms with fluorine atoms, thus creating many new products. Being a highly reactive element, fluorine forms compounds with several noble gases, as demonstrated by Neil Bartlett who synthesized xenon hexafluoroplatinate (XePtF6) in 1962. Fluorides of krypton and radon have also been prepared. Another compound is argon fluorohydride, but it is stable only at extremely low temperatures.
The following is a list of inorganic compounds of fluorine.
- Ammonium fluoride (NH4F)
- Antimony pentafluoride (SbF5)
- Boron trifluoride (BF3)
- Bromine pentafluoride (BrF5)
- Bromine trifluoride (BrF3)
- Cesium fluoride (CsF)
- Calcium fluoride (CaF2)
- Chlorine pentafluoride (ClF5)
- Fluorosulfuric acid (FSO3H)
- Hydrofluoric acid (HF)
- Iodine pentafluoride (IF5)
- Iodine heptafluoride (IF7)
- Lithium fluoride (LiF)
- Nitrogen trifluoride (NF3)
- Nitrosyl fluoride (NOF)
- Nitryl fluoride (NO2F)
- Phosphorus trifluoride (PF3)
- Phosphorus pentafluoride (PF5)
- Plutonium fluoride (PuF4)
- Potassium fluoride (KF)
- Radon difluoride (RnF2)
- Silver(I) fluoride (AgF)
- Silver(II) fluoride (AgF2)
- Sodium fluoride (NaF)
- Sulfur hexafluoride (SF6)
- Rubidium fluoride (RbF)
- Thionyl fluoride (SOF2)
- Tungsten(VI) fluoride (WF6)
- Uranium hexafluoride (UF6)
- Xenon hexafluoroplatinate (XePtF6)
- Xenon tetrafluoride (XeF4)
Applications
- Fluorine in the atomic and molecular states is used for plasma etching in the manufacture of semiconductors, flat panel displays, and MEMS (microelectromechanical systems).
- Hydrofluoric acid is used to etch glass in light bulbs and other products.
- Along with some of its compounds, fluorine is useful for the manufacture of pharmaceuticals, agrochemicals, lubricants, and textiles.
- Fluorine is used indirectly in the production of low-friction plastics such as Teflon.
- Fluorine has been used in producing halogenated alkanes (halons), which in turn have been used extensively in air conditioning and refrigeration. Chlorofluorocarbons (CFCs) have been banned for these applications because they contribute to expansion of the ozone hole in the upper atmosphere.
- Sulfur hexafluoride is an extremely inert, nontoxic gas, and a member of a class of compounds that are potent greenhouse gases.
- Many important agents for general anesthesia (such as sevoflurane, desflurane, and isoflurane) are derivatives of fluorohydrocarbons.
- Sodium hexafluoroaluminate (cryolite) is used in the electrolysis of aluminum.
- Compounds of fluorine, including sodium fluoride, are used in toothpaste to prevent dental cavities. These compounds are also added to municipal water supplies, a process called water fluoridation, but health concerns have led to controversy over this practice.
- At much higher concentrations, sodium fluoride has been used as an insecticide, especially against cockroaches.
- Fluorides have been used in the past to reduce the melting temperatures of metals and ores, and to help them flow.
- 18F, a radioactive isotope of fluorine with a half-life of 110 minutes, emits positrons and is often used in medical imaging by a technique known as positron emission tomography.
- Fluorine is used for the production of uranium hexafluoride, which in turn is used to separate uranium isotopes, as noted above.
See also
Notes
- ↑ Peter Meiers, “Discovery of fluorine,” Fluoride-History.de. Retrieved September 7, 2007.
ReferencesISBN links support NWE through referral fees
External links
All links retrieved March 28, 2024.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.