Difference between revisions of "Phenylalanine" - New World Encyclopedia
({{Contracted}}) |
|||
| Line 1: | Line 1: | ||
| − | {{Claimed}} | + | {{Claimed}}{{Contracted}} |
:'''''Phe''' redirects here. For the BitTorrent feature, see [[PHE]]. For the constellation, see [[Phoenix (constellation)]].'' | :'''''Phe''' redirects here. For the BitTorrent feature, see [[PHE]]. For the constellation, see [[Phoenix (constellation)]].'' | ||
<!-- Here is a table of data; skip past it to edit the text. —> | <!-- Here is a table of data; skip past it to edit the text. —> | ||
Revision as of 19:58, 15 June 2007
- Phe redirects here. For the BitTorrent feature, see PHE. For the constellation, see Phoenix (constellation).
| Phenylalanine | |
|---|---|
| Systematic name | 2-Amino-3-phenyl- propanoic acid |
| Abbreviations | Phe F |
| Chemical formula | C9H11NO2 |
| Molecular mass | 165.19 g mol-1 |
| Melting point | 283 °C |
| Density | 1.29 g cm-3 |
| Isoelectric point | 5.5 |
| pKa | 2.20 9.09 |
| PubChem | 994 |
| CAS number |
|
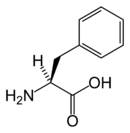
| SMILES | N[C@@H](Cc1ccccc1)C(O)=O |
| |
| Disclaimer and references | |
Phenyl alanine is an α-amino acid with the formula HO2CCH(NH2)CH2C6H5. This essential amino acid is classified as nonpolar because of the hydrophobic nature of the benyl side chain. The codons for L-phenylalanine are UUU and UUC. It is a white, powdery solid. L-Phenylalanine (LPA) is an electrically-neutral amino acid, one of the twenty common amino acids used to biochemically form proteins, coded for by DNA.
Biosynthesis
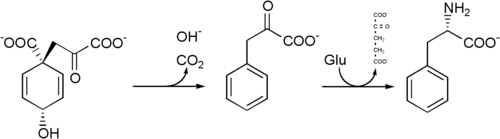
Phenylalanine cannot be made by animals, which have to obtain it from their diet. It is produced by plants and most microorganisms from prephenate, an intermediate on the shikimate pathway.[1]
Prephenate is decarboxylated with loss of the hydroxyl group to give phenylpyruvate. This species is transaminated using glutamate as the nitrogen source to give phenylalanine and α-ketoglutarate.
Other biological roles
L-phenylalanine can also be converted into L-tyrosine, another one of the DNA-encoded amino acids. L-tyrosine in turn is converted into L-DOPA, which is further converted into dopamine, norepinephrine (noradrenaline), and epinephrine (adrenaline) (the latter three are known as the catecholamines).
Phenylalanine uses the same active transport channel as tryptophan to cross the blood-brain barrier, and, in large quantities, interferes with the production of serotonin.
Lignin is derived from phenylalanine and from tyrosine. Phenylalanine is converted to cinnamic acid by the enzyme phenylalanine ammonia lyase.[1]
Phenylketonuria
The genetic disorder phenylketonuria (PKU) is the inability to metabolize phenylalanine. Individuals with this disorder are known as "phenylketonurics" and must abstain from consumption of phenylalanine. This dietary restriction also applies to pregnant women with hyperphenylalanine (high levels of phenylalanine in blood) because they do not properly metabolize the amino acid phenylalanine. Phenylalanine is present in many sugarless gums, Monster Munch crisps, sugarless soft drinks (such as Diet Coke, and Diet Pepsi), some forms of Lipton Tea, Icebreakers Mints, Clear Splash flavored water, and a number of other food products, all of which must be labeled: "Phenylketonurics: Contains phenylalanine." Phenylalanine itself is not present in the food. Rather, the artificial sweetener sold under the names "Equal" and "NutraSweet" contain aspartame, an ester that is hydrolyzed in the body to give phenylalanine, aspartic acid, and methanol (wood alcohol). Thus, aspartame is problematic for persons with PKU. The amounts produced by aspartame pose a risk however, as far larger quantities of the amino acid would be obtained through consuming normal protein. Interestingly, the macaque genome was recently sequenced and it was found that macaques naturally have a mutation that is found in humans who have PKU.[1]
Dietary aspects
Phenylalanine is contained in most protein-rich foods. Especially good sources are dairy products (curd, milk, cottage cheese), avocados, pulses and legumes (particularly peanuts and lima beans), nuts (pistachios, almonds), seeds (piyal seeds), leafy vegetables, whole grains, poultry, fish, other seafoods, and some diet beverages.
==D- and DL-phenylalanine==[citation needed] D-phenylalanine (DPA) either as a single enantiomer or as a component of the racemic mixture is available through conventional organic synthesis. It does not participate in protein biosynthesis although it is found in proteins, in small amounts, particularly aged proteins and food proteins that have been processed. The biological functions of D-amino acids remain unclear. Some D-amino acids, such as D-phenylalanine, may have pharmacologic activity. DL-Phenylalanine is marketed as a nutritional supplement for its putative analgesic and antidepressant activities. The putative analgesic activity of DL-phenylalanine may be explained by the possible blockage by D-phenylalanine of enkephalin degradation by the enzyme carboxypeptidase A. The mechanism of DL-phenylalanine's putative antidepressant activity may be accounted for by the precursor role of L-phenylalanine in the synthesis of the neurotransmitters norepinephrine and dopamine. Elevated brain norepinephrine and dopamine levels are thought to be associated with antidepressant effects.
D-phenylalanine is absorbed from the small intestine, following ingestion, and transported to the liver via the portal circulation. A fraction of D-phenylalanine appears to be converted to L-phenylalanine. D-phenylalanine is distributed to the various tissues of the body via the systemic circulation. D-phenylalanine appears to cross the blood-brain barrier with less efficiency than L-phenylalanine. A fraction of an ingested dose of D-phenylalanine is excreted in the urine.
History
The genetic codon for phenylalanine was the first to be discovered. Marshall W. Nirenberg discovered that insertion of m-RNA made up of multiple uracil repeats into E. coli, the bacterium produced a new protein, made up solely of repeated phenylalanine amino acids.
ReferencesISBN links support NWE through referral fees
- ↑ 1.0 1.1 Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
External links
- Phenylalanine and tyrosine biosynthesis
- Computational Chemistry Wiki
- Nitrogen Order's Molecule of the Week
- DL-phenylalanine versus imipramine in depression
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.