Lipid
Along with proteins, nucleic acids and carbohydrates, lipids are one of the major classes of biologically important molecules or biomolecules. They are water-insoluble organic compounds that are highly soluble in nonpolar organic solvents.
Although the term lipid is often used informally as a synonym for fat, the latter is in fact a subgroup of lipids referred to as a triglyceride. Unlike other groups of molecules, lipids comprise a broad and diverse range of structures, which also include phospholipids (components of cell membranes), sterols (most notably cholesterol and the steroid hormones), and more complex lipid derivatives such as glycolipids (sugar-linked lipids).
Consistent with their diverse chemical and structural properties, lipids have a variety of functions in the body:
- Structuring cell membranes. The cell membrane constitutes a barrier for the cell and controls the flow of material in and out of the cell.
- Energy storage. Triglycerides are an efficient form of energy storage that can be mobilized and broken down when fuel is needed.
- Signal transduction, or the transmission of information in cells. Lipid hormones like steroids and eicosanoids also mediate communication between cells.
- Cellular metabolism." Lipid vitamins, fat-soluble vitamins that include Vitamins A, D, E, and K, are required for metabolism, usually as coenzymes.
Despite the tainted reputation of certain lipids (most notably fats and cholesterol), many lipids are absolutely essential for life, playing a number of important roles in nutrition and health. However, abnormal levels of certain lipids, such as cholesterol and trans fatty acids are risk factors for heart disease and other diseases.
The major classes of lipids and their properties
The term lipid is really a catch-all phrase for a wide variety of hydrocarbon-based molecules of biological origin. Lipids encompass a huge range of structures, which can be aliphatic or aromatic, acyclic or cyclic, straight or branched, saturated or unsaturated, flexible or rigid. This diversity makes it impossible to define lipids on the basis of a single core structural feature or biosynthetic origin.
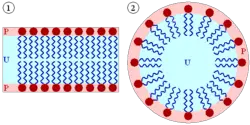
However, one shared property of many lipids is that they are amphipathic (or amphiphilic) molecules. Although lipids are predominantly nonpolar or hydrophobic ("water-fearing"), meaning that they do not interact well with polar solvents like water, most lipids also have some polar or hydrophilic ("water-loving") component. In the case of cholesterol, the polar group is a mere -OH (hydroxyl or alcohol). In the case of the membrane lipids called phospholipids, the polar groups are considerably larger and more polar.
This amphipathic character of many lipids, particularly the subgroup of phospholipids, directly influences their function in the body, by allowing them to spontaneously organize into cellular or intracellular compartments in water. Within the aqueous environment of the body, the polar heads of lipids tend to orient outward to interact with external water molecules, while the hydrophobic tails tend to minimize their contact with water. The nonpolar tails of lipids cluster together internally, forming a small sphere called a micelle, in the case of single-tailed amphipathic lipids. Two-tailed phospholipids instead form lipid bilayers, which can be considerably larger than micelles, and form a hollow sphere that encloses a separate aqueous compartment. These bilayers are the structural components of the biological membranes that form the biological membranes of cells as well as intracellular compartments called organelles.
The basic classes of lipids are as follows:
- Fatty acids: a type of carboxylic acid (an organic acid with terminal carboxyl group [-COOH]) that is a component of many other classes of lipids.
- Glycerides (or glycerolipids): structurally based on a glycerol backbone, these lipids include monoglycerides, diglycerides, and triglycerides as well as the phosphoglycerides (or glycerophospholipids) found in biological membranes.
- Nonglycerides: structurally based on a non-glycerol backbone, this category includes sphingolipids, [sterol]] lipids (such as cholesterol and the steroid hormones), prenol lipids (such as terpenoids), waxes, and polyketides.
- More complex lipid derivatives, such as sugar-linked lipids (glycolipids) and protein-linked lipids.
An alternative classification system has been proposed (J. Lipid Res. 46:839), which instead divides lipids into the following groups: (1) fatty acyls, (2) glycerolipids, (3) glycerophospholipids, (4) sphingolipids, (5) sterol lipids, (6) prenol lipids, (7) saccharolipids and (8) polyketides.
Fatty acids: the building blocks of lipids
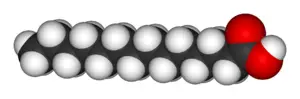
Fatty acids can be described as a class of compounds containing a long hydrocarbon chain and a terminal carboxylate group. They have a general structure of CH3(CH2)nCOOH. The chain usually ranges from 14 to 24 carbons in length, and typically contains an even number of carbons. Fatty acids can be either saturated or unsaturated:
- Saturated fatty acids have no double bonds between the carbon atoms of the fatty acid chain (hence, they are fully saturated with hydrogen atoms).
- Fatty acids with one or more double bonds are known as unsaturated fatty acids. The presence of double bonds generally reduces the melting point of fatty acids. Short chain length and unsaturation thus enhances the fluidity of fatty acids and their derivatives.
Unsaturated fatty acids can occur either in cis or trans geometric isomers. In most naturally occurring fatty acids, the double bonds are in the cis configuration. However, trans bonds are characteristically produced during the industrial hydrogenation of plant oils. Research suggests that, for reasons not yet well understood, increasing amounts of trans fats areo correlate with circulatory diseases such as atherosclerosis and coronary heart disease.
When they are not attached to other molecules, they are known as "free" fatty acids. Free fatty acids may come from the breakdown of a triglyceride into its components (fatty acids and glycerol). Free fatty acids are an important source of fuel for many tissues since they can yield relatively large quantities of ATP. Although many cell types can use either glucose or fatty acids for fuel, heart and skeletal muscle prefer fatty acids. On the other hand, the brain cannot use fatty acids as a source of fuel, relying instead on glucose, or on ketone bodies produced by the liver from fatty acid metabolism during starvation, or periods of low carbohydrate intake.
Glycerides: the energy storage lipids
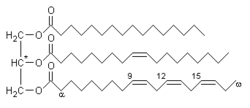
Glycerides have a glycerol core structure and one or more fatty acyl groups, which are fatty acid-derived chains attached to the glycerol backbone by ester linkages. Glycerides with three acyl groups (triglycerides or neutral fats) are the main storage form of fat in animals and plants. Triglycerides (which are also known as triacylglycerols or triacylglycerides) are stored in specialized cells called adipocytes, which compose the adipose tissue that cushions and insulates the body.
Triglycerides play an important role in metabolism as energy sources. They contain more than twice as much energy (9 kcal/g) as carbohydrates and proteins. Triglycerides were evolutionarily selected as the primary form of energy storage because their chemical properties make them a concentrated and efficient solution; they are reduced and anhydrous, as opposed to the more polar carbohydrates, which need to be stored with water.
Phospholipids: membrane components
Phospholipids are the major constituents of biological membranes, such as the cell's plasma membrane and the intracellular membranes of organelles. They are derived either from glycerol, a three-carbon alcohol, or sphingosine, a more complex alcohol. The former, called phosphoglycerides (or glycerophospholipids) consist of a glycerol backbone, two fatty acid chains, and a phosphorylated alcohol.
In addition to their structural described above, phospholipids also play a role in cell signaling: for instance, the polar head groups or fatty acid tails can be released from specific phospholipids through enzyme-catalyzed hydrolysis to generate the second messengers that are used in signal transduction to relay signals within a cell.
While phospholipids are the major component of biological membranes, other non-glyceride lipid components like sphingolipids and sterols (such as cholesterol in animal cell membranes) are also found in biological membranes.
Sterol lipids: structure and signaling
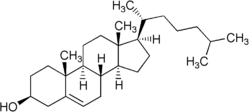
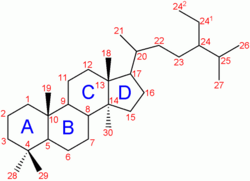
Cholesterol
Cholesterol is a sterol lipid (a combination steroid and alcohol) with the chemical formula C27H45OH. It is found in the cell membranes of all body tissues, and transported in the blood plasma of all animals. Lesser amounts of cholesterol are also found in plant membranes.
Cholesterol is an important component of cell membranes that enhances their fluidity. Cholesterol also aids in the manufacture of bile (which helps digest fats), and is also important for the metabolism of fat-soluble vitamins.
Cholesterol and triglycerides are transported in body fluids in the form of lipoprotein particles, the natural carrier molecules of the body, which are classified according to density. When doctors talk to their patients about the health concerns of cholesterol, they are often referring to "bad cholesterol", or low-density lipoprotein (LDL). "Good cholesterol" is high-density lipoprotein (HDL). Both types of cholesterol have roles in the body: LDL transports cholesterol to peripheral tissues and regulate the synthesis of cholesterol at these sites, while HDL "sweeps" the blood of cholesterol released into the plasma from dying cells and from membranes undergoing turnover (regeneration). However, high levels of LDL in the blood, due to the congenital absence or malfunciton of LDL receptors, or high dietary consumption of "bad cholesterol," may lead to the build-up of atherosclerotic plaques in arteries, which may in turn result in cardiovascular disease.
Steroid hormones
Cholesterol is an important precursor of steroid hormones, such as progesterone, testosterone, estradiol, and cortisol. Steroid hormones produce their physiological effects by binding to steroid hormone receptor proteins. The binding of steroid hormones to their receptors causes changes in gene transcription and cell function.
Some of the common categories of steroids include:
- Anabolic steroids are a class of steroids that interact with androgen receptors to increase muscle and bone synthesis. There are natural and synthetic anabolic steroids. These are the "steroids" used by athletes to increase performance.
- Corticosteroids include glucocorticoid and mineralocorticoids:
- Glucocorticoids regulate many aspects of metabolism and immune function, and often prescribed by doctors to reduce inflammatory conditions like asthma and arthritis.
- Mineralocorticoids are corticosteroids that help maintain blood volume and control renal excretion of electrolytes.
- Sex steroids are a subset of sex hormones that produce sex differences or support reproduction. They include androgens, estrogens, and progestagens.
ReferencesISBN links support NWE through referral fees
- Stryer, Lubert. 1994. Biochemistry, 4th edition. New York, NY: W.H. Freeman.
External links
- ApolloLipids - Provides dyslipidemia and cardiovascular disease prevention and treatment information as well as continuing medical education programs.
- Lipids, Membranes and Vesicle Trafficking - The Virtual Library of Biochemistry and Cell Biology
- The Lipid library - provides information on the chemistry, analysis and biochemistry of lipids
- LIPID MAPS: LIPID Metabolites and Pathways Strategy
- IUPAC glossary entry for the lipid class of molecules what is IUPAC?
- "A comprehensive classification system for lipids"
- CCMDWeb, a lipid health educational resource on global risk reduction for dyslipidemia and cardiovascular disease as well as online CME programs.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.