Difference between revisions of "Hydrogen peroxide" - New World Encyclopedia
| Line 268: | Line 268: | ||
==External links== | ==External links== | ||
| − | * [http://msds.fmc.com/msds/100000010225-MSDS_US-E.pdf Material Safety Data Sheet] | + | * [http://msds.fmc.com/msds/100000010225-MSDS_US-E.pdf Material Safety Data Sheet: Hydrogen Peroxide 20 to 40%] ''FMC.'' Retrieved December 7, 2007. |
| − | * [http://www.atsdr.cdc.gov/tfactsx4.html ATSDR Agency for Toxic Substances and Disease Registry FAQ] | + | * [http://www.atsdr.cdc.gov/tfactsx4.html ATSDR Agency for Toxic Substances and Disease Registry FAQ] Retrieved December 7, 2007. |
| − | * [http://www.erps.org Experimental Rocket Propulsion Society] | + | * [http://www.erps.org Experimental Rocket Propulsion Society] Retrieved December 7, 2007. |
| − | + | * [http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc01/icsc0164.htm International Chemical Safety Card 0164] Retrieved December 7, 2007. | |
| − | * [http://www.ilo.org/public/english/protection/safework/cis/products/icsc/dtasht/_icsc01/icsc0164.htm International Chemical Safety Card 0164] | + | * [http://www.cdc.gov/niosh/npg/npgd0335.html NIOSH Pocket Guide to Chemical Hazards] Retrieved December 7, 2007. |
| − | * [http://www.cdc.gov/niosh/npg/npgd0335.html NIOSH Pocket Guide to Chemical Hazards] | + | * [http://www.gkllc.com General Kinetics Inc. Hydrogen Peroxide Rocket Engines and Gas Generators] Retrieved December 7, 2007. |
| − | + | * [http://www.quackwatch.org/01QuackeryRelatedTopics/Cancer/oxygen.html Oxygenation Therapy: Unproven Treatments for Cancer and AIDS] Retrieved December 7, 2007. | |
| − | * [http://www.gkllc.com General Kinetics Inc. Hydrogen Peroxide Rocket Engines and Gas Generators] | + | * [http://news.bbc.co.uk/1/hi/england/london/4197500.stm Explosion of a lorry carrying hydrogen peroxide closes M25 motorway.] Retrieved December 7, 2007. |
| − | * [http://www.quackwatch.org/01QuackeryRelatedTopics/Cancer/oxygen.html Oxygenation Therapy:Unproven Treatments for Cancer and AIDS] | + | * [http://www.gaiaresearch.co.za/hydroperoxide.html Hydrogen Peroxide in the Human Body.] Retrieved December 7, 2007. |
| − | * [http://news.bbc.co.uk/1/hi/england/london/4197500.stm Explosion of a lorry carrying hydrogen peroxide closes M25 motorway.] | ||
| − | * [http://www.gaiaresearch.co.za/hydroperoxide.html Hydrogen Peroxide in the Human Body] | ||
[[Category:Physical sciences]] | [[Category:Physical sciences]] | ||
Revision as of 06:12, 7 December 2007
| Hydrogen peroxide | |
|---|---|
 | |
| General | |
| Systematic name | Dihydrogen dioxide |
| Other names | Hydrogen peroxide hydrogen dioxide dioxidane |
| Molecular formula | H2O2 |
| Molar mass | 34.0147 g·mol·−1. |
| Appearance | Very pale blue color; colorless in solution. |
| CAS number | [7722-84-1] [1] |
| Properties | |
| Density and phase | 1.4 g·cm−3, liquid |
| Solubility in water | Miscible. |
| Melting point | -11 °C (262.15 K) |
| Boiling point | 150.2 °C (423.35 K) |
| Acidity (pKa) | 11.65 |
| Viscosity | 1.245 cP at 20 °C |
| Structure | |
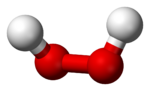
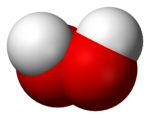
| Molecular shape | bent |
| Dipole moment | 2.26 D |
| Hazards | |
| MSDS | 30% hydrogen peroxide msds 60% hydrogen peroxide msds |
| Main hazards | Oxidant, corrosive. |
| NFPA 704 | |
| Flash point | Non-flammable. |
| R/S statement | R: R5, R8, R20, R22,R35 S: (S1), S2, S17, S26,S28, S36, S37, S39, S45 |
| RTECS number | MX0900000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | ? |
| Other cations | Sodium peroxide |
| Related compounds | Water ozone hydrazine |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Hydrogen peroxide (H2O2) is a very pale blue liquid which appears colorless in a dilute solution, slightly more viscous than water. It is a weak acid. It has strong oxidizing properties and is therefore a powerful bleaching agent that has found use as a disinfectant, as an oxidizer, and in rocketry (particularly in high concentrations as high-test peroxide (HTP) as a monopropellant), and in bipropellant systems.
History
Hydrogen peroxide was first isolated in 1818 by Louis Jacques Thénard by reacting barium peroxide with nitric acid. An improved version of this process used hydrochloric acid, followed by sulphuric acid to precipitate the barium sulfate byproduct. Thenard's process was used from the end of the nineteenth century until the middle of the twentieth century.[1] Modern production methods are discussed below.
Manufacture
Hydrogen peroxide is manufactured today almost exclusively by the autoxidation of 2-ethyl-9,10-dihydroxyanthracene to 2-ethylanthraquinone and hydrogen peroxide using oxygen from the air. The anthraquinone derivative is then extracted out and reduced back to the dihydroxy compound using hydrogen gas in the presence of a metal catalyst. The overall equation for the process is deceptively simple:
- H2 + O2 → H2O2
However the economics of the process depend on effective recycling of the quinone and extraction solvents, and of the hydrogenation catalyst.
Formerly inorganic processes were used, employing the electrolysis of an aqueous solution of sulfuric acid or acidic ammonium bisulfate (NH4HSO4), followed by hydrolysis of the peroxydisulfate ((SO4)2)2− which is formed.
Storage
Regulations vary, but low concentrations, such as 2.5% are widely available and legal to buy for medical use.
Hydrogen peroxide should be stored in a container made from a material that doesn't react or catalyse the chemical. Numerous materials and processes are available, some stainless steels, many plastics, glasses and some aluminum alloys are compatible.[2]
As peroxide is a strong oxidizer it should be stored away from fuel sources and sources of catalytic contamination (see decomposition section). Apart from obvious fire risks, peroxide vapor can react with hydrocarbons and alcohols to form contact explosives. Because oxygen is formed during natural decomposition of the peroxide, the resultant increase in pressure can cause a container (such as of glass) to shatter.
Peroxide should be kept cool, as peroxide vapor can detonate above 70 °C.
Deaths have occurred from storage in inadequately marked containers due to its apparent similarity to water.
Physical properties
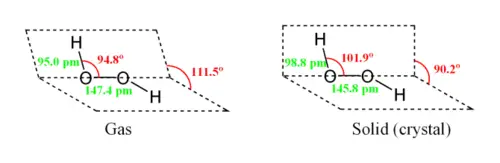
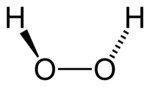
While the anti conformer would minimize steric repulsions, a 90° torsion angle would optimize mixing between the filled p-type orbital of the oxygen (one of the lone pairs) and the LUMO of the vicinal O-H bond.[3] Reflecting a compromise between the two interactions, gaseous and liquid hydrogen peroxide adopts an anticlinal "skewed" shape. This rotational conformation is a compromise between the anti conformer, which would minimize steric repulsion, and between the lone pairs on the oxygen atoms. Despite the fact that the O-O bond is a single bond, the molecule has a remarkably high barrier to complete rotation of 29.45 kJ/mol (compared with 12.5 kJ/mol for the rotational barrier of ethane). The increased barrier is also attributed to lone-pair lone-pair repulsion. The bond angles are affected by hydrogen bonding, which is relevant to the structural difference between gaseous and crystalline forms; indeed a wide range of values is seen in crystals containing molecular H2O2.
Chemical properties
H2O2 is one of the most powerful oxidizers known — stronger than chlorine, chlorine dioxide, and potassium permanganate. And through catalysis, H2O2 can be converted into hydroxyl radicals (.OH) with reactivity second only to fluorine.
| Oxidant | Oxidation potential, V |
|---|---|
| Fluorine | 3.0 |
| Hydroxyl radical | 2.8 |
| Ozone | 2.1 |
| Hydrogen peroxide | 1.8 |
| Potassium permanganate | 1.7 |
| Chlorine dioxide | 1.5 |
| Chlorine | 1.4 |
Hydrogen peroxide can decompose spontaneously into water and oxygen. It usually acts as an oxidizing agent, but there are many reactions where it acts as a reducing agent, releasing oxygen as a by-product.
It also readily forms both inorganic and organic peroxides.
Decomposition
Hydrogen peroxide always decomposes (disproportionates) exothermically into water and oxygen gas spontaneously:
- 2 H2O2 → 2 H2O + O2
This process is very favorable; it has a ΔHo of −98.2 kJ·mol−1 and a ΔGo of −119.2 kJ·mol−1 and a ΔS of 70.5 J·mol−1·K−1. The rate of decomposition is dependent on the temperature and concentration of the peroxide, as well as the pH and the presence of impurities and stabilizers. Hydrogen peroxide is incompatible with many substances that catalyse its decomposition, including most of the transition metals and their compounds. Common catalysts include manganese dioxide, and silver. The same reaction is catalyzed by the enzyme catalase, found in the liver, whose main function in the body is the removal of toxic byproducts of metabolism and the reduction of oxidative stress. The decomposition occurs more rapidly in alkali, so acid is often added as a stabilizer.
The liberation of oxygen and energy in the decomposition has dangerous side effects. Spilling high concentration peroxide on a flammable substance can cause an immediate fire, which is further fueled by the oxygen released by the decomposing hydrogen peroxide. High-strength peroxide (also called high-test peroxide, or HTP) must be stored in a suitable,[citation needed] vented container to prevent the buildup of oxygen gas, which would otherwise lead to the eventual rupture of the container.
In the presence of certain catalysts, such as Fe2+ or Ti3+, the decomposition may take a different path, with free radicals such as HO· (hydroxyl) and HOO· being formed. A combination of H2O2 and Fe2+ is known as Fenton's reagent.
A common concentration for hydrogen peroxide is "20 volume," which means that when 1 volume of hydrogen peroxide is decomposed, it produces 20 volumes of oxygen. This is equivalent to about 6% or 1.7M.
The hydrogen peroxide you buy at the drug store is a three percent solution. In such small amounts, it is less stable, decomposing faster, but it is stabilized with acetanilide, a substance that has toxic side effects in significant amounts.
Redox reactions
In aqueous solution, hydrogen peroxide can oxidize or reduce a variety of inorganic ions. When it acts as a reducing agent, oxygen gas is also produced. In acid solution Fe2+ is oxidized to Fe3+,
- [[2 Fe2+]](aq) + H2O2 + 2 H+(aq) → 2 [[Fe3+]](aq) + 2H2O(l)
and sulfite (SO32−) is oxidized to sulfate (SO42−). However, potassium permanganate is reduced to Mn2+ by acidic H2O2. Under alkaline conditions, however, some of these reactions reverse; for example, Mn2+ is oxidized to Mn4+ (as MnO2).
Another example of hydrogen peroxide acting as a reducing agent is the reaction with Sodium hypochlorite, this is a convenient method for preparing oxygen in the laboratory.
NaOCl + H2O2 → O2 + NaCl + H2O
Hydrogen peroxide is frequently used as an oxidizing agent in organic chemistry. One application is for the oxidation of thioethers to sulfoxides.[citation needed] For example, methyl phenyl sulfide was oxidised to methyl phenyl sulfoxide in 99% yield in methanol in 18 hours (or 20 minutes using a TiCl3 catalyst):
- Ph-S-CH3 + H2O2 → Ph-S(O)-CH3 + H2O
Alkaline hydrogen peroxide is used for epoxidation of electron-deficient alkenes such as acrylic acids, and also for oxidation of alkylboranes to alcohols, the second step of hydroboration-oxidation.
Formation of peroxide compounds
Hydrogen peroxide is a weak acid, and it can form hydroperoxide or peroxide salts or derivatives of many metals.
For example, on addition to an aqueous solution of chromic acid (CrO3) or acidic solutions of dichromate salts, it will form an unstable blue peroxide CrO(O2)2. In aqueous solution it rapidly decomposes to form oxygen gas and chromium salts.
It can also produce peroxoanions by reaction with anions; for example, reaction with borax leads to sodium perborate, a bleach used in laundry detergents:
- Na2B4O7 + 4 H2O2 + 2 NaOH → 2 Na2B2O4(OH)4 + H2O
H2O2 converts carboxylic acids (RCOOH) into peroxy acids (RCOOOH), which are themselves used as oxidizing agents. Hydrogen peroxide reacts with acetone to form acetone peroxide, and it interacts with ozone to form hydrogen trioxide. Reaction with urea produces carbamide peroxide, used for whitening teeth. An acid-base adduct with triphenylphosphine oxide is a useful "carrier" for H2O2 in some reactions.
Hydrogen peroxide reacts with ozone to form trioxidane.
Alkalinity
Hydrogen peroxide is a much weaker base than water, but it can still form adducts with very strong acids. The superacid HF/SbF5 forms unstable compounds containing the [H3O2]+ ion.
Concentration
Hydrogen peroxide works best as a propellant in extremely high concentrations— roughly over 70%. Although any concentration of peroxide will generate some hot gas (oxygen plus some steam), at concentrations above approximately 67%, the heat of decomposing hydrogen peroxide becomes large enough to completely vaporize all the liquid at standard temperature. This represents a safety and utilization turning point, since decomposition of any concentration above this amount is capable of transforming the liquid entirely to heated gas (the higher the concentration, the hotter the resulting gas). This very hot steam/oxygen mixture can then be used to generate maximal thrust, power, or work, but it also makes explosive decomposition of the material far more hazardous.
Normal propellant grade concentrations therefore vary from 70 to 98%, with common grades of 70, 85, 90, and 98%. Many of these grades and variations are described in detail in the United States propellant specification number MIL-P-16005 Revision F, which is currently available. The available suppliers of high concentration propellant grade hydrogen peroxide are generally one of the large commercial companies which make other grades of hydrogen peroxide; including Solvay Interox, FMC, and Degussa. Other companies which have made propellant grade hydrogen peroxide in the recent past include Air Liquide and DuPont. DuPont recently sold its hydrogen peroxide manufacturing business to Degussa.
Propellant-grade hydrogen peroxide is available to qualified buyers. Typically this chemical is only sold to commercial companies or government institutions which have the ability to properly handle and utilize the material. Non-professionals have purchased 70% or lower concentration hydrogen peroxide (the remaining 30% is water with traces of impurities and stabilizing materials, such as tin salts, phosphates, nitrates, and other chemical additives), and increased its concentration themselves. Many amateurs try distillation, but this is extremely dangerous with hydrogen peroxide; peroxide vapor can ignite or detonate depending on specific combinations of temperature and pressure. In general any boiling mass of high concentration hydrogen peroxide at ambient pressure will produce vapor phase hydrogen peroxide which can detonate. This hazard is mitigated, but not entirely eliminated with vacuum distillation. Other approaches for concentrating hydrogen peroxide are sparging and fractional crystallization.
High concentration hydrogen peroxide is readily available in 70, 90, and 98% concentrations in sizes of 1 gallon, 30 gallon, and bulk tanker truck volumes. Propellant grade hydrogen peroxide is being used on current military systems and is in numerous defense and aerospace research and development programs. Many privately funded rocket companies are using hydrogen peroxide, notably Blue Origin, and some amateur groups have expressed interest in manufacturing their own peroxide, for their use and for sale in small quantities to others.
Hazards
Hydrogen peroxide, either in pure or diluted form, can pose several risks:
- Above roughly 70 percent concentrations, hydrogen peroxide can give off vapor that can detonate above 70 °C (158 °F) at normal atmospheric pressure. This can then cause a boiling liquid expanding vapor explosion (BLEVE) of the remaining liquid. Distillation of hydrogen peroxide at normal pressures is thus highly dangerous.
- Hydrogen peroxide vapors can form sensitive contact explosives with hydrocarbons such as greases. Hazardous reactions ranging from ignition to explosion have been reported with alcohols, ketones, carboxylic acids (particularly acetic acid), amines and phosphorus.[citation needed] The saying is 'peroxides kill chemists'.[citation needed]
- Hydrogen peroxide, if spilled on clothing (or other flammable materials), will preferentially evaporate water until the concentration reaches sufficient strength, then clothing will spontaneously ignite. Leather generally contains metal ions from the tanning process and will often catch fire almost immediately.[4]
- Concentrated hydrogen peroxide (at concentration exceeding 50%) is corrosive, and even domestic-strength solutions can cause irritation to the eyes, mucous membranes and skin.[5] Swallowing hydrogen peroxide solutions is particularly dangerous, as decomposition in the stomach releases large quantities of gas (10 times the volume of a 3% solution) leading to internal bleeding. Inhaling over 10% can cause severe pulmonary irritation.[citation needed]
Hydrogen peroxide is naturally produced as a byproduct of oxygen metabolism, and virtually all organisms possess enzymes known as peroxidases, which apparently harmlessly catalytically decomposes low concentrations of hydrogen peroxide to water and oxygen (see Decomposition above).
In one incident, several people were injured after a hydrogen peroxide spill on board Northwest Airlines Flight 957 because they mistook it for water.[6]
An MSDS will contain more information on the risks of working with this chemical.
See also
Notes
- ↑ Jones, Craig W. 1999. Applications of Hydrogen Peroxide and Derivatives. RSC Clean Technology Monographs. Cambridge, UK: Royal Society of Chemistry. ISBN 0854045368.
- ↑ Material Compatibility with Hydrogen Peroxide. OzoneLab. Retrieved December 7, 2007.
- ↑ Anslyn, Eric V., and Dennis A. Dougherty. 2004. Modern Physical Organic Chemistry. Sausalito, CA: University Science. p. 122. ISBN 1891389319.
- ↑ Material Tests with HTP Armadilloaerospace. Retrieved December 7, 2007.
- ↑ For example, see an MSDS: Hydrogen Peroxide Solution 3%. J.T. Baker. Retrieved December 7, 2007.
- ↑ Hazardous Materials Incident Brief DCA-99-MZ-001, "Spill of undeclared shipment of hazardous materials in cargo compartment of aircraft." National Transportation Safety Board. October 28, 1998; adopted May 17, 2000. Retrieved December 7, 2007.
ReferencesISBN links support NWE through referral fees
- J. Drabowicz et al., in The Syntheses of Sulphones, Sulphoxides and Cyclic Sulphides, p112-116, G. Capozzi et al., eds., John Wiley & Sons, Chichester, UK, 1994. ISBN 0-471-93970-6.
- N. N. Greenwood, A. Earnshaw, Chemistry of the Elements, 2nd ed., Butterworth-Heinemann, Oxford, UK, 1997. A great description of properties & chemistry of H2O2.
- J. March, Advanced Organic Chemistry, 4th ed., p. 723, Wiley, New York, 1992.
- W. T. Hess, W.T. 1995. Hydrogen Peroxide, in Kirk-Othmer Encyclopedia of Chemical Technology, Vol. 13, 4th ed. New York: Wiley, 961-995.
External links
- Material Safety Data Sheet: Hydrogen Peroxide 20 to 40% FMC. Retrieved December 7, 2007.
- ATSDR Agency for Toxic Substances and Disease Registry FAQ Retrieved December 7, 2007.
- Experimental Rocket Propulsion Society Retrieved December 7, 2007.
- International Chemical Safety Card 0164 Retrieved December 7, 2007.
- NIOSH Pocket Guide to Chemical Hazards Retrieved December 7, 2007.
- General Kinetics Inc. Hydrogen Peroxide Rocket Engines and Gas Generators Retrieved December 7, 2007.
- Oxygenation Therapy: Unproven Treatments for Cancer and AIDS Retrieved December 7, 2007.
- Explosion of a lorry carrying hydrogen peroxide closes M25 motorway. Retrieved December 7, 2007.
- Hydrogen Peroxide in the Human Body. Retrieved December 7, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.