Biological pest control
Biological control of pests in agriculture is a method of controlling pests (including insects, mites, weeds, and plant diseases) that relies on predation, parasitism, herbivory, or other natural mechanisms. It can be an important component of integrated pest management (IPM) programs.
Overview
Biological Control is defined as the reduction of pest populations by natural enemies and typically involves an active human role. Natural enemies of insect pests, also known as biological control agents, include predators, parasitoids, and pathogens. Biological control agents of plant diseases are most often referred to as antagonists. Biological control agents of weeds include herbivores and plant pathogens. Predators, such as lady beetles and lacewings, are mainly free-living species that consume a large number of prey during their lifetime. Parasitoids are species whose immature stage develops on or within a single insect host, ultimately killing the host. Most have a very narrow host range. Many species of wasps and some flies are parasitoids. Pathogens are disease-causing organisms including bacteria, fungi, and viruses. They kill or debilitate their host and are relatively specific to certain insect groups. There are three basic types of biological control strategies; conservation, classical biological control, and augmentation. These are discussed in more detail below.
Conservation
The conservation of natural enemies is probably the most important and readily available biological control practice available to homeowners and gardeners. Natural enemies occur in all areas, from the backyard garden to the commercial field. They are adapted to the local environment and to the target pest, and their conservation is generally simple and cost-effective. Lacewings, lady beetles, hover fly larvae, and parasitized aphid mummies are almost always present in aphid colonies. Fungus-infected adult flies are often common following periods of high humidity. These naturally occurring biological controls are often susceptible to the same pesticides used to target their hosts. Preventing the accidental eradication of natural enemies is termed simple conservation.
Classical Biological Control
Classical biological control is the introduction of natural enemies to a new locale where they did not originate or do not occur naturally. This is usually done by government authorities. In many instances the complex of natural enemies associated with an insect pest may be inadequate. This is especially evident when an insect pest is accidentally introduced into a new geographic area without its associated natural enemies. These introduced pests are referred to as exotic pests and comprise about 40% of the insect pests in the United States. Examples of introduced vegetable pests include the European corn borer, one of the most destructive insects in North America. To obtain the needed natural enemies, scientists turned to classical biological control. This is the practice of importing, and releasing for establishment, natural enemies to control an introduced (exotic) pest, although it is also practiced against native insect pests. The first step in the process is to determine the origin of the introduced pest and then collect appropriate natural enemies associated with the pest or closely related species. The natural enemy is then passed through a rigorous quarantine process, to ensure that no unwanted organisms (such as hyperparasitoids) are introduced, then they are mass produced, and released. Follow-up studies are conducted to determine if the natural enemy becomes successfully established at the site of release, and to assess the long-term benefit of its presence.
There are many examples of successful classical biological control programs. One of the earliest successes was with the cottony cushion scale, a pest that was devastating the California citrus industry in the late 1800s. A predatory insect, the vedalia beetle, and a parasitoid fly were introduced from Australia. Within a few years the cottony cushion scale was completely controlled by these introduced natural enemies. Damage from the alfalfa weevil, a serious introduced pest of forage, was substantially reduced by the introduction of several natural enemies. About 20 years after their introduction, the alfalfa area treated for alfalfa weevil in the northeastern United States was reduced by 75 percent. A small wasp, Trichogramma ostriniae, introduced from China to help control the European corn borer, is a recent example of a long history of classical biological control efforts for this major pest. Many classical biological control programs for insect pests and weeds are under way across the United States and Canada.
Classical biological control is long lasting and inexpensive. Other than the initial costs of collection, importation, and rearing, little expense is incurred. When a natural enemy is successfully established it rarely requires additional input and it continues to kill the pest with no direct help from humans and at no cost. Unfortunately, classical biological control does not always work. It is usually most effective against exotic pests and less so against native insect pests. The reasons for failure are often not known, but may include the release of too few individuals, poor adaptation of the natural enemy to environmental conditions at the release location, and lack of synchrony between the life cycle of the natural enemy and host pest.
Augmentation
This third type of biological control involves the supplemental release of natural enemies. Relatively few natural enemies may be released at a critical time of the season (inoculative release) or literally millions may be released (inundative release). Additionally, the cropping system may be modified to favor or augment the natural enemies. This latter practice is frequently referred to as habitat manipulation.
An example of inoculative release occurs in greenhouse production of several crops. Periodic releases of the parasitoid, Encarsia formosa, are used to control greenhouse whitefly, and the predaceous mite, Phytoseiulus persimilis, is used for control of the two-spotted spider mite.
Lady beetles, lacewings, or parasitoids such as Trichogramma are frequently released in large numbers (inundative release). Recommended release rates for Trichogramma in vegetable or field crops range from 5,000 to 200,000 per acre per week depending on level of pest infestation. Similarly, entomopathogenic nematodes are released at rates of millions and even billions per acre for control of certain soil-dwelling insect pests.
Habitat or environmental manipulation is another form of augmentation. This tactic involves altering the cropping system to augment or enhance the effectiveness of a natural enemy. Many adult parasitoids and predators benefit from sources of nectar and the protection provided by refuges such as hedgerows, cover crops, and weedy borders.
Mixed plantings and the provision of flowering borders can increase the diversity of habitats and provide shelter and alternative food sources. They are easily incorporated into home gardens and even small-scale commercial plantings, but are more difficult to accommodate in large-scale crop production. There may also be some conflict with pest control for the large producer because of the difficulty of targeting the pest species and the use of refuges by the pest insects as well as natural enemies.
Examples of habitat manipulation include growing flowering plants (pollen and nectar sources) near crops to attract and maintain populations of natural enemies. For example, hover fly adults can be attracted to umbelliferous plants in bloom.
Biological control experts in California have demonstrated that planting prune trees in grape vineyards provides an improved overwintering habitat or refuge for a key grape pest parasitoid. The prune trees harbor an alternate host for the parasitoid, which could previously overwinter only at great distances from most vineyards. Caution should be used with this tactic because some plants attractive to natural enemies may also be hosts for certain plant diseases, especially plant viruses that could be vectored by insect pests to the crop. Although the tactic appears to hold much promise, only a few examples have been adequately researched and developed.
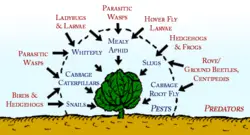
Examples of predators

Ladybugs, and in particular their larvae which are active between May and July in the northern hemisphere, are voracious predators of aphids such as greenfly and blackfly, and will also consume mites, scale insects and small caterpillars. The ladybug is a very familiar beetle with various colored markings, whilst its larvae are initially small and spidery, growing up to 17 mm long. The larvae have a tapering segmented grey/black body with orange/yellow markings nettles in the garden and by leaving hollow stems and some plant debris over-winter so that they can hibernate over winter.
Hoverflies. Resembling slightly darker bees or wasps, they have characteristic hovering, darting flight patterns. There are over 100 species of hoverfly whose larvae principally feed upon greenfly, one larva devouring up to fifty a day, or 1000 in its lifetime. They also eat fruit tree spider mites and small caterpillars. Adults feed on nectar and pollen, which they require for egg production. Eggs are minute (1 mm), pale yellow white and laid singly near greenfly colonies. Larvae are 8-17 mm long, disguised to resemble bird droppings, they are legless and have no distinct head. Semi-transparent in a range of colours from green, white, brown and black.
Hoverflies can be encouraged by growing attractant flowers such as the poached egg plant (Limnanthes douglasii), marigolds or phacelia throughout the growing season.
Dragonflies are important predators of mosquitoes, both in the water, where the dragonfly naiads eat mosquito larvae, and in the air, where adult dragonflies capture and eat adult mosquitoes. Community-wide mosquito control programs that spray adult mosquitoes also kill dragonflies, thus removing an important biocontrol agent, and can actually increase mosquito populations in the long term.
Other useful garden predators include lacewings, pirate bugs, rove and ground beetles, aphid midge, centipedes, predatory mites, as well as larger fauna such as frogs, toads, lizards, hedgehogs, slow-worms and birds. Cats and rat terriers kill field mice, rats, june bugs, and birds. Dogs chase away many types of pest animals. Dachshunds are bred specifically to fit inside tunnels underground to kill badgers.
Parasitic insects
Most insect parasitoids are wasps or flies. Parasitiods comprise a diverse range of insects that lay their eggs on or in the body of an insect host, which is then used as a food for developing larvae. Parasitic wasps take much longer than predators to consume their victims, for if the larvae were to eat too fast they would run out of food before they became adults. Such parasites are very useful in the organic garden, for they are very efficient hunters, always at work searching for pest invaders. As adults they require high energy fuel as they fly from place to place, and feed upon nectar, pollen and sap, therefore planting plenty of flowering plants, particularly buckwheat, umbellifers, and composites will encourage their presence.
Four of the most important groups are:
- Ichneumonid wasps: (5-10 mm). Prey mainly on caterpillars of butterflies and moths.
- Braconid wasps: Tiny wasps (up to 5 mm) attack caterpillars and a wide range of other insects including greenfly. A common parasite of the cabbage white caterpillar- seen as clusters of sulphur yellow cocoons bursting from collapsed caterpillar skin.
- Chalcid wasps: Among the smallest of insects (<3 mm). Parasitize eggs/larvae of greenfly, whitefly, cabbage caterpillars, scale insects and strawberry tortrix moth.
- Tachinid flies: Parasitize a wide range of insects including caterpillars, adult and larval beetles, true bugs, and others.
Plants to regulate insect pests
Choosing a diverse range of plants for the garden can help to regulate pests in a variety of ways, including;
- Masking the crop plants from pests, depending on the proximity of the companion or intercrop.
- Producing olfactory inhibitors, odors that confuse and deter pests.
- Acting as trap plants by providing an alluring food that entices pests away from crops.
- Serving as nursery plants, providing breeding grounds for beneficial insects.
- Providing an alternative habitat, usually in a form of a shelterbelt, hedgerow, or beetle bank where beneficial insects can live and reproduce. Nectar-rich plants that bloom for long periods are especially good, as many beneficials are nectivorous during the adult stage, but parasitic or predatory as larvae. A good example of this is the soldier beetle which is frequently found on flowers as an adult, but whose larvae eat aphids, caterpillars, grasshopper eggs, and other beetles.
The following are plants often used in vegetable gardens to deter insects [1]
| Plant | Pests |
|---|---|
| Basil | Repels flies and mosquitoes. |
| Catnip | Deters flea beetle. |
| Garlic | Deters Japanese beetle. |
| Horseradish | Deters potato bugs. |
| Marigold | The workhorse of pest deterrents. Discourages Mexican bean beetles, nematodes and others. |
| Mint | Deters white cabbage moth, ants. |
| Nasturtium | Deters aphids, squash bugs and striped pumpkin beetles. |
| Pot Marigold | Deters asparagus beetles, tomato worm, and general garden pests. |
| Peppermint | Repels the white cabbage butterfly. |
| Rosemary | Deters cabbage moth, bean beetles and carrot fly. |
| Sage | Deters cabbage moth and carrot fly. |
| Southernwood | Deters cabbage moth. |
| Summer Savory | Deters bean beetles. |
| Tansy | Deters flying insects, Japanese beetles, striped cucumber beetles, squash bugs and ants. |
| Thyme | Deters cabbage worm. |
| Wormwood | Deters animals from garden. |
Directly introducing biological controls
Most of the biological controls listed above depend on providing incentives in order to 'naturally' attract beneficial insects to the garden. However there are occasions when biological controls can be directly introduced. Common biocontrol agents include parasitoids, predators, pathogens or weed feeders. This is particularly appropriate in situations such as the greenhouse, a largely artificial environment, and are usually purchased by mail order.
Some biocontrol agents that can be introduced include;
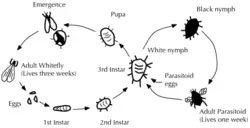
- Encarsia formosa. This is a small predatory chalcid wasp which is parasitical on whitefly, a sap-feeding insect which can cause wilting and black sooty moulds. It is most effective when dealing with low level infestations, giving protection over a long period of time. The wasp lays its eggs in young whitefly 'scales', turning them black as the parasite larvae pupates. It should be introduced as soon as possible after the first adult whitefly are seen. Should be used in conjunction with insecticidal soap.
- Red spider mite, another pest found in the greenhouse, can be controlled with the predatory mite Phytoseilus persimilis. This is slightly larger than its prey and has an orange body. It develops from egg to adult twice as fast as the red spider mite and once established quickly overcomes infestation.
- A fairly recent development in the control of slugs is the introduction of 'Nemaslug', a microscopic nematode (Phasmarhabditis hermaphrodita) which will seek out and Parasitize slugs, reproducing inside them and killing them. The nematode is applied by watering onto moist soil, and gives protection for up to six weeks in optimum conditions, though is mainly effective with small and young slugs under the soil surface.
- A bacterial biological control which can be introduced in order to control butterfly caterpillars is Bacillus thuringiensis. This available in sachets of dried spores which are mixed with water and sprayed onto vulnerable plants such as brassicas and fruit trees. The bacterial disease will kill the caterpillars, but leave other insects unharmed. There are strains of Bt that are effective against other insect larvae. Bt israelensis is effective against mosquito larvae and some midges.
- A biological control being developed for use in the treatment of plant disease is the fungus Trichoderma viride. This has been used against Dutch Elm disease, and to treat the spread of fungal and bacterial growth on tree wounds. It may also have potential as a means of combating silver leaf disease.
- The parasitoid Gonatocerus ashmeadi (Hymenoptera: Mymaridae) has been introduced to control the glassy-winged sharpshooter Homalodisca vitripennis (Hemipterae: Cicadellidae) in French Polynesia and has successfully controlled ~95% of the pest density[2]
Economics of biological pest control
Biological control proves to be very successful economically, and even when the method has been less successful, it still produces a benefit-to-cost ratio of 11:1. One study has estimated that a successful biocontrol program returns £32 in benefits for each £1 invested in developing and implementing the program, i.e., a 32:1 benefit-to-cost ratio. The same study had shown that an average chemical pesticide program only returned profits in the ratio of 13:1.[citation needed]
Negative results of biological pest control
In some cases, biological pest control can have unforeseen negative results, that could outweigh all benefits. For example, when the mongoose was introduced to Hawaii in order to control the rat population, it predated on the endemic birds of Hawaii, especially their eggs, more often than it ate the rats.
ReferencesISBN links support NWE through referral fees
- ↑ Notes on Natural Pest Control for an Organic Garden - DigGood.com.
- ↑ Hoddle M.S., Grandgirard J., Petit J., Roderick G.K., Davies N. (2006). Glassy-winged sharpshooter Ko'ed - First round - in French Polynesia. Biocontrol News and Information 27 (3): 47N-62N.
See also
- Insectary plants
- Integrated Pest Management
- Japanese beetle (article includes information on biological control methods)
- Organic farming
- Pesticide
- Sterile insect technique
External links and references
- Biological Control: A Guide to Natural Enemies in North America
- Beyond Pesticides - Provides information on pesticides and alternatives to their use.
- Association of Natural Biocontrol Producers (ANBP) Trade association of biological pest control industry.
- Successful biocontrol of the GWSS in French Polynesia
- R. James Cook (September 1993). Making Greater Use of Introduced Microorganisms for Biological Control of Plant Pathogens. Annual Review of Phytopathology 31: 53-80.
- U.S. Congress, Office of Technology Assessment (1995). "Biologically based technologies for pest control". US Government Printing Office, Washington, DC.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.