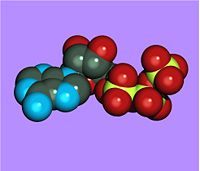
Adenosine triphosphate
| Adenosine 5'-triphosphate | |
|---|---|
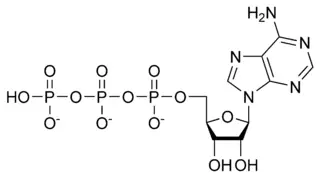
| |
| Chemical name |
[[[5-(6-aminopurin-9-yl)-3,4-dihydroxy-oxolan-2-yl] methoxy-hydroxy-phosphoryl] oxy-hydroxy-phosphoryl] oxyphosphonic acid |
| Abbreviations | ATP |
| Chemical formula | C10H16N5O13P3 |
| Molecular mass | 507.181 g mol-1 |
| Melting point | ? °C |
| Density | ? g/cm3 |
| pKa | ? |
| CAS number | 56-65-5 |
| EINECS | 200-283-2 |
| PubChem | 5957 |
Adenosine triphosphate (ATP) is the chemical compound known in biochemistry as the "molecular currency" of intracellular energy transfer; that is, ATP is able to store and transport chemical energy within cells. All cells&mdashprokaryote, animal, and plant&mdashuse ATP as the principal energy-carrying molecule and as the main energy source for driving energy-requiring reactions (endergonic); as such, ATP has been referred to as "life's energy currency" (Luria, Gould and Singer 1981).
In short, living cells require energy to survive and function, and most of this energy comes either via radiant energy from sunlight or from chemical energy tied up in interatomic bonds of nutrient molecules. When nutrient molecules coming from carbohydrates, fats, and proteins are oxidized by cells, a portion of the free energy released can be captured in the chemical bonds of ATP. ATP allows the storing of energy in cells as chemical potential and the circulation and use of this energy by the cell.
In addition to its energy-related function, ATP also plays an important role in the synthesis of nucleic acids. In signal transduction pathways, ATP is used to provide the phosphate for the protein-kinase reactions.
The ubiqutous presence of ATP in the cells of all living organisms indicates that it must have appeared early in the history of cellular life. The intricacy of how ATP is used by the body in various metabolic pathways, and its fundamental importance to living systems, reveals the complex interaction between parts of living systems.
Chemical properties
ATP consists of adenosine and three phosphate groups(triphosphate). The phosphoryl groups, starting with that on AMP, are referred to as the alpha (α), beta (β), and gamma (γ) phosphates. ATP is extremely rich in chemical energy, in particular between the second and third phosphate groups. The net change in energy of the decomposition of ATP into ADP and an inorganic phosphate is -12 kCal / mole in vivo, or inside of a living cell, and -7.3 kCal / mole in vitro, or in laboratory conditions. This massive release in energy makes the decomposition of ATP extremely exergonic, and hence useful as a means for chemically storing energy.
Synthesis
ATP can be produced by various cellular processes: Under aerobic conditions, the synthesis occurs in mitochondria during the oxidative phosphorylation and is catalyzed by ATP synthase; to a lesser degree, under anaerobic conditions, this is done through substrate phosphorylation catalyzed by two enzymes: PGK and Pyruvate kinase.
ATP is also synthesized through several so-called "replenishment" reactions catalyzed by the enzyme families of NDKs (Nucleoside diphosphate kinases), which use other nucleoside triphosphates as a high-energy phosphate donor, and the ATP:guanido-phosphotransferase family, which uses creatine.
- ADP + GTP ATP + GDP
In plants, ATP is synthesized in chloroplasts by photosynthesis during the light reactions of photosynthesis. However, this ATP is then used to power the Calvin cycle step of photosynthesis and so photosynthesis does not result in an overall production of ATP.
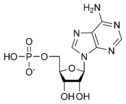 Adenosine monophosphate AMP |
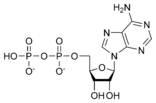 Adenosine diphosphate ADP |
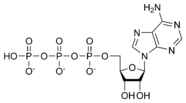 Adenosine triphosphate ATP |
The main fuels for ATP synthesis are Glc and FAs. First, Glc is broken down into pyruvate in the cytosol. Two molecules of ATP are generated for each molecule of Glc. The terminal stages of ATP synthesis are carried out in the mitochondrion and can generate up to 36 ATP, which is 40% energy efficiency.
Function
ATP energy is released when hydrolysis of the phosphate-phosphate bonds is carried out. This energy can be used by a variety of enzymes, motor proteins, and transport proteins to carry out the work of the cell. Also, the hydrolysis yields free inorganic Pi and ADP, which can be broken down further to another Pi and AMP. ATP can also be broken down to AMP directly, with the formation of PPi. This last reaction has the advantage of being an effectively irreversible process in aqueous solution.
ATP in the human body
The total quantity of ATP in the human body is about 0.1 mole. The energy used by human cells requires the hydrolysis of 200 to 300 moles of ATP daily. This means that each ATP molecule is recycled 2000 to 3000 times during a single day. ATP cannot be stored, hence its consumption must closely follow its synthesis. On a per-hour basis, 1 kilogram of ATP is created, processed and then recycled in the body.
Other uses
There is talk of using ATP as a power source for nanotechnology and implants. Artificial pacemakers could become independent of batteries. ATP is also present as a neurotransmitter independent from its energy-containing function. Receptors that utilise ATP as their ligand are known as purinoceptors.
See also
- Adenosine diphosphate (ADP)
- Adenosine monophosphate (AMP)
- Cyclic adenosine monophosphate (cAMP)
- ATPases
- ATP hydrolysis
- Citric acid cycle (also called the Krebs cycle or TCA cycle)
- Phosphagen
- Thioesters are related to ATP
- ATP thermochemistry
- Nucleotide exchange factor
External link
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - mtDNA - Oligonucleotide |
ar:ATP cs:Adenozin trifosfát da:ATP (kemi) de:Adenosintriphosphat es:Adenosín trifosfato fr:Adénosine triphosphate ko:아데노신 삼인산 id:ATP he:ATP lt:ATP lb:Adenosintriphosphat nl:Adenosinetrifosfaat ja:アデノシン三リン酸 pl:ATP pt:Adenosina tri-fosfato ru:Аденозинтрифосфорная кислота sl:Adenozintrifosfat sr:Аденозин трифосфат su:Adénosin trifosfat fi:ATP sv:Adenosintrifosfat zh:三磷酸腺苷
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.