Lichen

A lichen is a composite organism composed of a fungus (the mycobiont) in a symbiotic relationship with a photosynthetic partner (the photobiont, also known as the phycobiont) that can produce food for the lichen from sunlight. The photobiont is usually either green algae or cyanobacteria. A few lichens are known to contain yellow-green algae or, in one case, a brown alga. Some lichens contain both green algae and cyanobacteria as photobionts; in these cases, the cyanobacteria symbiont component may specialize in fixing atmospheric nitrogen for metabolic use.
The body (thallus) of most lichens is quite different from that of either the fungus or alga growing separately, and may strikingly resemble simple plants in form and growth (Sanders 2001). The fungus surrounds the algal cells, often enclosing them within complex fungal tissues unique to lichen associations; however, the algal cells are never enclosed inside the fungal cells themselves. The fungus may or may not penetrate into the algal cells with fine hyphal protrusions.
There are thousands of species of lichens, which are typically hardy, slow-growing organisms. They often are pioneer forms of life that can grow in harsh (extremes of temperature) environments, such as the arctic, or sparse environments, such as on rocks or in deserts. Many grow on the trunks of trees. They are a key food resource for caribou in the far north. As organisms that are very sensitive to pollutants, lichens are a good indicator species for environmental problems.
Harmony is seen in the cooperative relationship of two very different organisms, fungi and algae‚ÄĒso much so that they make one functioning organism. This fits with the view of Lynn Margulis that "Life did not take over the globe by combat, but by networking" (Margulis and Sagan 1986)‚ÄĒin other words, by cooperation.
Symbiotic relationship
The algal or cyanobacterial cells are photosynthetic, and as in higher plants they reduce atmospheric carbon dioxide into organic carbon sugars to feed both symbionts. Both partners gain water and mineral nutrients mainly from the atmosphere, through rain and dust. The fungal partner protects the alga by retaining water, serving as a larger capture area for mineral nutrients and, in some cases, provides minerals obtained from the substratum. If a cyanobacterium is present, as a primary partner or another symbiont in addition to green alga as in certain tripartite lichens, they can fix atmospheric nitrogen‚ÄĒcomplementing the activities of the green alga in tripartite lichens.
In general, the symbiosis involved in lichens is considered obligatory for successful growth and reproduction of the fungus; however, the significance for the algal symbiont is less clear. For some algae, the symbiosis may be obligatory for survival in a particular habitat; in other cases, the symbiosis might not be advantageous for the alga.
There is some evidence to suggest that the lichen symbiosis is parasitic rather than mutualistic (Ahmadjian 1993), with lichens involving a controlled form of parasitism of algal cells. For example, photobiont cells are routinely destroyed in the course of nutrient exchange. The association is able to continue because photobiont cells reproduce faster than they are destroyed (Ahmadjian 1993). Also, in another indication of possibly a parasitic relationship, in laboratory settings cyanobacteria grow faster when they are alone rather than when they are part of a lichen.
Thus, there is some controversy as to whether the lichen symbiosis should be considered an example of mutualism or parasitism or commensalism. Nonetheless, the lichen is typically a highly stable association that probably extends the ecological range of both partners. There is also a mutualistic component to the relationship: The fungus part of the lichen provides the alga with water and minerals that the fungus absorbs from whatever the lichen is growing on, its substrate. As for the alga, it uses the minerals and water to make food for the fungus and itself.
Types
Lichens take the external shape of the fungal partner and hence are named based on the fungus. The fungus most commonly forms the majority of a lichen's bulk, though in filamentous and gelatinous lichens this may not always be the case. The lichen fungus is typically a member of the Ascomycota‚ÄĒrarely a member of the Basidiomycota, and then termed basidiolichens to differentiate them from the more common ascolichens.
Formerly, some lichen taxonomists placed lichens in their own division, the Mycophycophyta, but this practice is no longer accepted because the components belong to separate lineages. Neither the ascolichens nor the basidiolichens form monophyletic lineages in their respective fungal phyla, but they do form several major solely or primarily lichen-forming groups within each phylum (Lutzoni 2004). Even more unusual than basidiolichens is the fungus Geosiphon pyriforme, a member of the Glomeromycota that is unique in that it encloses a cyanobacterial symbiont inside its cells. Geospihon is not usually considered to be a lichen, and its peculiar symbiosis was not recognized for many years. The genus is more closely allied to endomycorrhizal genera.
Growth form
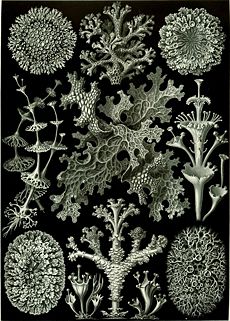
Lichens are informally classified by growth form into:
- Crustose (paint-like, flat), e.g., Caloplaca flavescens
- Filamentose (hair-like), e.g., Ephebe lanata
- Foliose (leafy), e.g., Hypogymnia physodes
- Fruticose (branched), e.g., Cladina evensii, C. subtenuis, and Usnea australis
- Leprose (powdery), e.g., Lepraria incana
- Squamulose (consisting of small scale-like structures, lacking a lower cortex), e.g., Normandina pulchella
- Gelatinous lichens, in which the cyanobacteria produce a polysaccharide that absorbs and retains water.
Morphology and structure
Some lichens have the aspect of leaves (foliose lichens); others cover the substratum like a crust (crustose lichens); others adopt shrubby forms (fruticose lichens); and there are gelatinous lichens.
Although the form of a lichen is determined by the genetic material of the fungal partner, association with a photobiont is required for the development of that form. When grown in the laboratory in the absence of its photobiont, a lichen fungus develops as an undifferentiated mass of hyphae. If combined with its photobiont under appropriate conditions, its characteristic form emerges in the process called morphogenesis (Brodo et al. 2001). In a few remarkable cases, a single lichen fungus can develop into two very different lichen forms when associating with either a green algal or a cyanobacterial symbiont. Quite naturally, these alternative forms were at first considered to be different species, until they were first found growing in a conjoined manner.
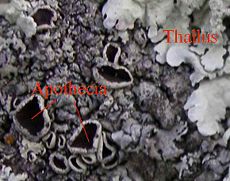
Under magnification, a section through a typical foliose lichen thallus reveals four layers of interlaced fungal filaments. The uppermost layer is formed by densely agglutinated fungal hyphae building a protective outer layer called the cortex. In lichens that include both green algal and cyanobacterial symbionts, the cyanobacteria may be held on the upper or lower surface in small pustules called cephalodia/cephalodium. Beneath the upper cortex is an algal layer composed of algal cells embedded in rather densely interwoven fungal hyphae. Each cell or group of cells of the photobiont is usually individually wrapped by hyphae, and in some cases penetrated by an haustorium. Beneath this algal layer is a third layer of loosely interwoven fungal hyphae without algal cells. This layer is called the medulla. Beneath the medulla, the bottom surface resembles the upper surface and is called the lower cortex, again consisting of densely packed fungal hyphae. The lower cortex often bears root-like fungal structures known as rhizines, which serve to attach the thallus to the substrate on which it grows.
Lichens sometimes also contain structures made from fungal metabolites, for example crustose lichens sometimes have a polysaccharide layer in the cortex. Although each lichen thallus generally appears homogeneous, some evidence seems to suggest that the fungal component may consist of more than one genetic individual of that species. This seems to also be true of the photobiont species involved.
Reproduction
Many lichens reproduce asexually, either by vegetative reproduction or through the dispersal of diaspores containing algal and fungal cells. Soredia (singular soredium) are small groups of algal cells surrounded by fungal filaments that form in structures called soralia, from which the soredia can be dispersed by wind. Another form of diaspore are isidia, elongated outgrowths from the thallus that break off for mechanical dispersal. Fruticose lichens in particular can easily fragment. Due to the relative lack of differentiation in the thallus, the line between diaspore formation and vegetative reproduction is often blurred. Many lichens break up into fragments when they dry, dispersing themselves by wind action, to resume growth when moisture returns.
Many lichen fungi appear to reproduce sexually in a manner typical of fungi, producing spores that are presumably the result of sexual fusion and meiosis. Following dispersal, such fungal spores must meet with a compatible algal partner before a functional lichen can form. This may be a common form of reproduction in basidiolichens, which form fruitbodies resembling their nonlichenized relatives. Among the ascolichens, spores are produced in spore-producing bodies, the three most common spore body types are the apothecia, perithecia, and the pycnidia.
Ecology
Lichens are often the first to settle in places lacking soil, constituting the sole vegetation in some extreme environments, such as those found at high mountain elevations and at high latitudes. Some survive in the tough conditions of deserts, and others on frozen soil of the arctic regions. Recent ESA research shows that lichen can even endure extended exposure to space.
Lichens must compete with plants for access to sunlight, but because of their small size and slow growth, they thrive in places where higher plants have difficulty growing.
A major ecophysiological advantage of lichens is that they are poikilohydric (poikilo‚ÄĒvariable, hydric‚ÄĒrelating to water), meaning that though they have little control over the status of their hydration, they can tolerate irregular and extended periods of severe desiccation. Like some mosses, liverworts, ferns, and a few "resurrection plants," upon desiccation, lichens enter a metabolic suspension or stasis (known as cryptobiosis) in which the cells of the lichen symbionts are dehydrated to a degree that halts most biochemical activity. In this cryptobiotic state, lichens can survive wider extremes of temperature, radiation, and drought in the harsh environments they often inhabit.
Lichens do not have roots and do not need to tap continuous reservoirs of water like most higher plants. Thus, they can grow in locations impossible for most plants, such as bare rock, sterile soil or sand, and various artificial structures such as walls, roofs, and monuments. Many lichens also grow as epiphytes (epi‚ÄĒon the surface, phyte‚ÄĒplant) on other plants, particularly on the trunks and branches of trees. When growing on other plants, lichens are not parasites; they do not consume any part of the plant nor poison it. Some ground-dwelling lichens, such as members of genus Cladina (reindeer lichens), however, produce chemicals which leach into the soil and inhibit the germination of plant seeds and growth of young plants.
Stability (that is, longevity) of their substratum is a major factor of lichen habitats. Most lichens grow on stable rock surfaces or the bark of old trees, but many others grow on soil and sand. In these latter cases, lichens are often an important part of soil stabilization; indeed, in some desert ecosystems, vascular (higher) plant seeds cannot become established except in places where lichen crusts stabilize the sand and help retain water.
When growing on mineral surfaces, some lichens slowly decompose their substrate by chemically degrading and physically disrupting the minerals, contributing to the process of weathering by which rocks are gradually turned into soil. While this contribution to weathering is usually benign, it can cause problems for artificial stone structures. For example, there is an ongoing lichen growth problem on Mount Rushmore National Memorial that requires the employment of mountain-climbing conservators to clean the monument.
Lichens may be eaten by some animals, such as reindeer, living in arctic regions. The larvae of a surprising number of Lepidoptera species feed exclusively on lichens. These include Common Footman and Marbled Beauty. However, lichens are very low in protein and high in carbohydrates, making them unsuitable for some animals. Lichens are also used by the Northern Flying Squirrel for nesting, food, and a water source during winter.
Although lichens typically grow in naturally harsh environments, most lichens, especially epiphytic fruticose species and those containing cyanobacteria, are sensitive to manufactured pollutants and to air quality. Hence, they have been widely used as pollution indicator organisms.
Many lichens produce secondary compounds, including pigments that reduce harmful amounts of sunlight and powerful toxins that reduce herbivory or kill bacteria. These compounds are very useful for lichen identification, and have (or had) economic importance as dyes or primitive antibiotics. Extracts from many Usnea species were used to treat wounds in Russia in the mid-twentieth century (Kane 2002). Orcein and other lichen dyes have largely been replaced by synthetic versions (Armstrong 2007).
The European Space Agency has discovered that lichens can survive unprotected in space (ESA 2005; Young 2005). In an experiment led by Leopoldo Sancho from the Complutense University of Madrid, two species of lichen ‚ÄĒRhizocarpon geographicum and Xanthoria elegans‚ÄĒwere sealed in a capsule and launched on a Russian Soyuz rocket on May 31, 2005. Once in orbit, the capsules were opened and the lichens were directly exposed to the vacuum of space with its widely fluctuating temperatures and cosmic radiation. After 15 days, the lichens were brought back to earth and were found to be in full health with no discernible damage from their time in orbit.
Gallery
ReferencesISBN links support NWE through referral fees
- Ahmadjian, V. 1993. The Lichen Symbiosis. New York: John Wiley & Sons. ISBN 0471578851
- Armstrong, W. P. 2007. Lichen dyes and perfumes. Waynesword. Retrieved October 5, 2007.
- British Broadcasting Corporation (BBC). 2006. Insight into sex life of lichens. Retrieved October 5, 2007.
- Brodo, I. M., S. D. Sharnoff, and S. Sharnoff. 2001. Lichens of North America. New Haven: Yale University Press. ISBN 0300082495
- European Space Agency (ESA). 2005. Lichens survive in space. Retrieved October 5, 2007.
- Gilbert, O. 2004. The Lichen Hunters. The Book Guild Ltd. ISBN 1857769309
- Hawksworth, D. L. and M. R. D. Seaward. 1977. Lichenology in the British Isles 1568-1975. Richmond, Surrey: The Richmond Publishing Co. ISBN 0855462000
- Kane, C. W. 2002. Usnea]. Tuscon Clinic of Botanical Medicine Newsletter 4(4). Retrieved October 5, 2007.
- Knowles, M. C. 1929. "The lichens of Ireland." Proceedings of the Royal Irish Academy 38: 1-32.
- Lutzoni, et al. 2004. Assembling the fungal tree of life: Progress, classification, and evolution of subcellular traits. Amer J Bot 91: 1446-1480.
- Margulis L. and D. Sagan. 1986. Microcosmos. New York: Summit Books. ISBN 0671441698
- Purvis, O. W., B. J. Coppins, D. L. Hawksworth, P. W. James, and D. M. Moore. (Eds.). 1994. The lichen flora of Great Britain and Ireland. The Lichenologist 26(2): 217-223.
- Sanders, W. B. 2001. Lichens: interface between mycology and plant morphology. Bioscience 51: 1025-1035.
- Seaward, M. R. D. 1984. Census catalogue of Irish lichens. Glasra 8: 1-32.
- Young, K. 2005. Hardy lichen shown to survive in space. New Scientist November 10, 2005. Retrieved October 5, 2007.
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.