Biotin
| Biotin | |
|---|---|
| |
| General | |
| Systematic name | |
| Chemical formula | C10H16N2O3S |
| Molecular weight | 244.31 g/mol |
| Other names |
|
| Vitamin properties | |
| Solubility | Water |
| RDA (adult male) | 30 ”g/day |
| RDA (adult female) | 30 ”g/day |
| RDA upper limit (adult male) | None |
| RDA upper limit (adult female) | None |
| Deficiency symptoms |
|
| Excess symptoms | None |
| Dietary sources | |
| Infobox disclaimer and references | |
Biotin, also known as vitamin B7 or vitamin H, is one of the B vitamins, a group of chemically distinct, water-soluble vitamins that also includes thiamine, riboflavin, niacin, pantothenic acid, pyridoxine, folic acid, and others. Vitamins are organic (carbon-containing) nutrients obtained through the diet and essential in small amounts for normal metabolic reactions in humans. The B vitamins (vitamin B complex) were once considered to be a single vitamin, like vitamin C. However, vitamin B is now seen as a complex of different vitamins that generally are found in the same foods.
Biotin is important in a number of essential metabolic reactions in humans, including catalyzing the synthesis of fatty acids, metabolism of the amino acid leucine, and gluconeogenesis (generation of glucose from non-sugar carbon substrates like pyruvate, glycerol, and amino acids). Biotin is important in cell growth; plays a role in the Krebs cycle, which is the biochemical pathway in which energy is released from food (glucose, amino acids, and fat); helps with the transfer of carbon dioxide; and is useful in maintaining a steady blood sugar level.
A harmonious relationship with symbiotic bacteria in the intestine of humans helps in preventing biotin deficiency as these bacteria synthesize small amounts of biotin. On the other hand, biotin reveals the importance of balance in one's diet, as excessive consumption of raw egg-whites over a long period of time can result in biotin deficiency, as a protein in the egg-whites binds with biotin and results in its removal.
Structure
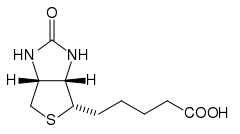
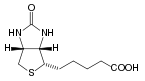
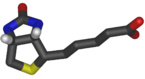
Biotin has the chemical formula C10H16N2O3S.
Biotin is composed of an ureido (tetrahydroimidizalone) ring fused with a tetrahydrothiophene ring, which is an organic compound consisting of a five-membered ring containing four carbon atoms and a sulfur atom. A valeric acid substituentâstraight chain alkyl carboxylic acid with the chemical formula CH3(CH2)3COOH)âis attached to one of the carbon atoms of the tetrahydrothiophene ring.
Biotin deficiency
Biotin deficiency is a rare metabolic genetic disorder. For that reason, statutory agencies in many countries (e.g., the Australian Department of Health and Aging) do not prescribe a recommended daily intake. Biotin deficiency can have a very serious, even fatal, outcome if it is allowed to progress without treatment. Signs and symptoms of biotin deficiency can develop in persons of any age, race, or gender.
Biotin deficiency rarely occurs in healthy individuals, since the daily requirements of biotin are low, many foods contain adequate amounts, intestinal bacteria synthesize small amounts, and the body effectively scavenges and recycles biotin from bodily waste. However, deficiency can be caused by excessive consumption of raw egg-whites over a long period (months to years). Egg-whites contain high levels of avidin, a protein that binds biotin strongly. Once a biotin-avidin complex forms, the bond is essentially irreversible. The biotin-avidin complex is not broken down nor liberated during digestion, and the biotin-avidin complex is lost in the feces. Once cooked, the egg-white avidin becomes denatured and entirely non-toxic.
Initial symptoms of biotin deficiency include:
- Dry skin
- Seborrheic dermatitis
- Fungal infections
- Rashes including erythematous periorofacial macular rash
- Fine and brittle hair
- Hair loss or total alopecia
If left untreated, neurological symptoms can develop, including:
- Mild depression, which may progress to profound lassitude and, eventually, to somnolence
- Changes in mental status
- Generalized muscular pains (myalgias)
- Hyperesthesias and paresthesias
The treatment for biotin deficiency is to simply start taking some biotin supplements.
Uses
Biotin supplements are often recommended as a natural product to counteract the problem of hair loss in both children and adults. There are, however, no studies that show any benefit in any case where the subject is not actually biotin deficient. The signs and symptoms of biotin deficiency include hair loss that progresses in severity to include loss of eye lashes and eye brows in severely deficient subjects. Some shampoos are available that contain biotin, but it is doubtful whether they would have any useful effect, as biotin is not absorbed well through the skin.
Biotin is often recommended for strengthening hair and nails. Consequently, it is found in many cosmetic and health products for the hair and skin.
Children with a rare inherited metabolic disorder called phenylketonuria (PKU; in which one is unable to break down the amino acid phenylalanine) often develop skin conditions such as eczema and seborrheic dermatitis in areas of the body other than the scalp. The scaly skin changes that occur in people with PKU may be related to poor ability to use biotin. Increasing dietary biotin has been known to improve seborrheic dermatitis in these cases.
People with type 2 diabetes often have low levels of biotin. Biotin may be involved in the synthesis and release of insulin. Preliminary studies in both animals and people suggest that biotin may help improve blood sugar control in those with diabetes, particularly type 2 diabetes.
Biochemistry
Biotin is a cofactor responsible for carbon dioxide transfer in several carboxylase enzymes:
- Acetyl-CoA carboxylase alpha
- Acetyl-CoA carboxylase beta
- Methylcrotonyl-CoA carboxylase
- Propionyl-CoA carboxylase
- Pyruvate carboxylase
The attachment of biotin to various chemical sites, called biotinylation, can be used as an important laboratory technique to study various processes including DNA transcription and replication. Biotin itself is known to biotinylate histones, but is not found naturally on DNA.
Biotin binds very tightly to the tetrameric protein streptavidin, with a dissociation constant Kd in the order of 10-15 mol/L (Bonjour 1977, Green 1975) or 4x10-14 (Holmberg et al. 2005). Holmberg et al. (2005) note that the biotin-streptavidin system is the strongest noncovalent biological interaction known. This is often used in different biotechnological applications. Holmberg et al. showed how to utilize high temperatures to efficiently break the interaction without denaturation of the streptavidin.
In the biology laboratory, biotin is sometimes chemically linked, or tagged, to a molecule or protein for biochemical assays. The specificity of the biotin-streptavidin linkage allow use in molecular, immunological, and cellular assays (Holmberg et al. 2005). Since avidin and streptavidin bind preferentially to biotin, biotin-tagged molecules can be extracted from a sample by mixing them with beads covered with avidin or strepavidin, and washing away anything unbound to the beads.
For example, biotin can be tagged onto a molecule of interest (e.g. protein), and this modified molecule will be mixed with a complex mixture of proteins. Avidin or streptavidin beads are added to the mixture, and the biotinylated molecule will bind to the beads. Any other proteins binding to the biotinylated molecule will also stay with the beads. All other unbound proteins can be washed away, and the scientist can use a variety of methods to determine which proteins have bound to the biotinylated molecule.
Biotinylated antibodies are used to capture avidin or streptavidin both the ELISPOT technique (Enzyme-Linked Immunosorbent SPOT, a method for monitoring immune responses in humans and animals) and the ELISA technique (Enzyme-Linked ImmunoSorbent Assay, a biochemical technique used in immunology to detect the presence of an antibody or an antigen in a sample).
ReferencesISBN links support NWE through referral fees
- Bonjour, J. R. 1977. Biotin in man's nutrition and therapy: A review. Int. J. Vitam. Nutr. Res. 47:107.
- Green, N. M. 1975. Biotin. Adv Protein Chem. 29: 85-133.
- Holmberg, A., A. Blomstergren, O. Nord, M. Lukacs, J. Lundeberg, and M. Uhlen. 2005. The biotin-streptavidin interaction can be reversibly broken using water at elevated temperatures. Electrophoresis 26(3): 501-10.
- Sloan, H. R., S. B. Freilich, and N. S. Scheinfeld. 2006. Biotin deficiency. eMedicine. Retrieved March 14, 2007.
| Vitamins |
|---|
| All B vitamins | All D vitamins |
| Retinol (A) | Thiamine (B1) | Riboflavin (B2) | Niacin (B3) | Pantothenic acid (B5) | Pyridoxine (B6) | Biotin (B7) | Folic acid (B9) | Cyanocobalamin (B12) | Ascorbic acid (C) | Ergocalciferol (D2) | Cholecalciferol (D3) | Tocopherol (E) | Naphthoquinone (K) |
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.