Difference between revisions of "Threonine" - New World Encyclopedia
(New page: {{Claimed}}) |
|||
| Line 1: | Line 1: | ||
{{Claimed}} | {{Claimed}} | ||
| + | <!-- Here is a table of data; skip past it to edit the text. —> | ||
| + | <!-- Submit {{:subst:chembox_simple_organic}} to get this template or go to [[:Template:Chembox_simple_organic]]. —> | ||
| + | {| id="bioChemInfoBox" align="right" border="1" cellspacing="0" cellpadding="3" style="margin: 0 0 0 0.5em; background: #FFFFFF; border-collapse: collapse; border-color: #C0C090; width: 320px; float: right;" | ||
| + | ! {{chembox header}}| '''Threonine''' | ||
| + | |- | ||
| + | | style="width: 30%;" | [[IUPAC nomenclature|Systematic name]] | ||
| + | | (2''S'',3''R'')-2-Amino-<br/>3-hydroxybutanoic acid | ||
| + | |- | ||
| + | | Abbreviations | ||
| + | | '''Thr<br/>T''' | ||
| + | |- | ||
| + | | [[Chemical formula]] | ||
| + | | C<sub>4</sub>H<sub>9</sub>NO<sub>3</sub> | ||
| + | |- | ||
| + | | [[Molecular mass]] | ||
| + | | 119.12 g mol<sup>-1</sup> | ||
| + | |- | ||
| + | | [[Melting point]] | ||
| + | | 256 °C | ||
| + | |- | ||
| + | | [[Density]] | ||
| + | | ? g cm<sup>-3</sup> | ||
| + | |- | ||
| + | | [[Isoelectric point]] | ||
| + | | 5.60 | ||
| + | |- | ||
| + | | [[Acid dissociation constant|p''K''<sub>a</sub>]] | ||
| + | | 2.20<br/>8.96 | ||
| + | |- | ||
| + | |[[PubChem]] | ||
| + | | [http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6288 6288] | ||
| + | |- | ||
| + | | [[CAS registry number|CAS number]] | ||
| + | | [72-19-5] | ||
| + | |- | ||
| + | | [[EINECS number]] | ||
| + | | 200-774-1 | ||
| + | |- | ||
| + | | {{chembox SMILES|value=C[C@@H](O)[C@H](N)C(O)=O}} | ||
| + | |- | ||
| + | | align="center" colspan="2" | [[Image:L-threonine-skeletal.png|100px|Chemical structure of Threonine]][[Image:L-threonine-3D-sticks.png|100px|Chemical structure of {{PAGENAME}}]] | ||
| + | |- | ||
| + | | {{chembox header}} | <small>[[wikipedia:Chemical infobox|Disclaimer and references]]</small> | ||
| + | |- | ||
| + | |} | ||
| + | |||
| + | '''Threonine''' is an α-[[amino acid]] with the [[chemical formula]] HO<sub>2</sub>CCH(NH<sub>2</sub>)CH(OH)CH<sub>3</sub>. Its three letter code is thr, its one letter code is T, and its [[codon]]s are ACU and ACA. This [[essential amino acid]] is classified as [[polar]]. Together with [[serine]] and [[tyrosine]], threonine is one of three proteinogenic amino acids bearing an alcohol group. | ||
| + | |||
| + | The threonine residue is susceptible to numerous [[posttranslational modification]]s. The hydroxy [[side chain]] can undergo O-linked [[glycosylation]]. Additionally, threonine residues undergo [[phosphorylation]] through the action of a threonine [[kinase]]. In its phosphorylated form, it can be referred to as phosphothreonine. | ||
| + | |||
| + | ====Allo-threonine==== | ||
| + | With two [[Chirality (chemistry)|chiral]] centers, threonine can exist in four possible [[stereoisomer]]s, or two possible [[diastereomer]]s of <small>L</small>-threonine. However, the name <small>L</small>-threonine is used for one single [[enantiomer]], (2''S'',3''R'')-2-amino-3-hydroxybutanoic acid. The second diastereomer (2''S'',3''S''), which is rarely present in nature, is called <small>L</small>-''allo''-threonine. | ||
| + | |||
| + | ==Biosynthesis== | ||
| + | As an essential amino acid, threonine is not synthesized in humans, hence we must ingest threonine or, more commonly, threonine-containing proteins. In plants and microorganisms, threonine is synthesized from [[aspartic acid]] via α-aspartyl-semialdehyde and [[homoserine]]. Homoserine undergoes ''O''-phosphorylation; this phosphate [[ester]] undergoes hydrolysis concomitant with relocation of the OH group.<ref>Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.</ref> Enzymes involved in a typical biosynthesis of threonine include: | ||
| + | # [[aspartokinase]] | ||
| + | # α-aspartate semialdehyde [[dehydrogenase]] | ||
| + | # homoserine dehydrogenase | ||
| + | # homoserine [[kinase]] | ||
| + | # threonine [[synthase]] | ||
| + | |||
| + | [[Image:Thr biosynthesis.gif|thumb|center|600px|Threonine biosynthesis]] | ||
| + | |||
| + | ==Metabolism== | ||
| + | Threonine is metabolized in two ways: | ||
| + | * It is converted to [[pyruvate]] | ||
| + | * It is converted to [[alpha-ketobutyrate]], and thereby enter the pathway leading to [[succinyl CoA]]. | ||
| + | |||
| + | ==Synthesis== | ||
| + | [[Racemic]] threonine can be prepared from [[crotonic acid]] by alpha-functionalization using [[mercury(II) acetate]].<ref>{{OrgSynth | author = Carter, H. E.; West, H. D. | title = dl-Threonine | collvol = 3 | collvolpages = 813 | year = 1955 | prep = cv3p0813}}</ref> | ||
| + | |||
| + | ==Sources== | ||
| + | Foods high in threonine include [[cottage cheese]], [[poultry]], [[fish]], [[meat]], [[lentil]]s, and sesame seeds.{{Fact|date=June 2007}} | ||
| + | |||
| + | ==References== | ||
| + | <references/> | ||
| + | == External links == | ||
| + | *[http://www.chem.qmul.ac.uk/iubmb/enzyme/reaction/AminoAcid/Thr.html Threonine biosynthesis] | ||
| + | *[http://www.compchemwiki.org/index.php?title=Threonine Computational Chemistry Wiki] | ||
| + | *[http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=205 CID 205] | ||
| + | *[http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6288 CID 6288] | ||
| + | |||
| + | {{ChemicalSources}} | ||
| + | |||
| + | {{AminoAcids}} | ||
| + | |||
| + | [[Category:Proteinogenic amino acids]] | ||
| + | [[Category:Glucogenic amino acids]] | ||
| + | [[Category:Ketogenic amino acids]] | ||
| + | [[Category:Essential amino acids]] | ||
| + | {{credit|137181091}} | ||
| + | [[Category:Life sciences]] | ||
Revision as of 15:42, 15 June 2007
| Threonine | |
|---|---|
| Systematic name | (2S,3R)-2-Amino- 3-hydroxybutanoic acid |
| Abbreviations | Thr T |
| Chemical formula | C4H9NO3 |
| Molecular mass | 119.12 g mol-1 |
| Melting point | 256 °C |
| Density | ? g cm-3 |
| Isoelectric point | 5.60 |
| pKa | 2.20 8.96 |
| PubChem | 6288 |
| CAS number | [72-19-5] |
| EINECS number | 200-774-1 |
| SMILES | C[C@@H](O)[C@H](N)C(O)=O |
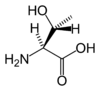 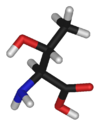
| |
| Disclaimer and references | |
Threonine is an α-amino acid with the chemical formula HO2CCH(NH2)CH(OH)CH3. Its three letter code is thr, its one letter code is T, and its codons are ACU and ACA. This essential amino acid is classified as polar. Together with serine and tyrosine, threonine is one of three proteinogenic amino acids bearing an alcohol group.
The threonine residue is susceptible to numerous posttranslational modifications. The hydroxy side chain can undergo O-linked glycosylation. Additionally, threonine residues undergo phosphorylation through the action of a threonine kinase. In its phosphorylated form, it can be referred to as phosphothreonine.
Allo-threonine
With two chiral centers, threonine can exist in four possible stereoisomers, or two possible diastereomers of L-threonine. However, the name L-threonine is used for one single enantiomer, (2S,3R)-2-amino-3-hydroxybutanoic acid. The second diastereomer (2S,3S), which is rarely present in nature, is called L-allo-threonine.
Biosynthesis
As an essential amino acid, threonine is not synthesized in humans, hence we must ingest threonine or, more commonly, threonine-containing proteins. In plants and microorganisms, threonine is synthesized from aspartic acid via α-aspartyl-semialdehyde and homoserine. Homoserine undergoes O-phosphorylation; this phosphate ester undergoes hydrolysis concomitant with relocation of the OH group.[1] Enzymes involved in a typical biosynthesis of threonine include:
- aspartokinase
- α-aspartate semialdehyde dehydrogenase
- homoserine dehydrogenase
- homoserine kinase
- threonine synthase
Metabolism
Threonine is metabolized in two ways:
- It is converted to pyruvate
- It is converted to alpha-ketobutyrate, and thereby enter the pathway leading to succinyl CoA.
Synthesis
Racemic threonine can be prepared from crotonic acid by alpha-functionalization using mercury(II) acetate.[2]
Sources
Foods high in threonine include cottage cheese, poultry, fish, meat, lentils, and sesame seeds.[citation needed]
ReferencesISBN links support NWE through referral fees
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- ↑ Carter, H. E.; West, H. D. (1955). "dl-Threonine". Org. Synth.; Coll. Vol. 3: 813.
External links
Template:ChemicalSources
| Major families of biochemicals | ||
| Peptides | Amino acids | Nucleic acids | Carbohydrates | Nucleotide sugars | Lipids | Terpenes | Carotenoids | Tetrapyrroles | Enzyme cofactors | Steroids | Flavonoids | Alkaloids | Polyketides | Glycosides | ||
| Analogues of nucleic acids: | The 20 Common Amino Acids | Analogues of nucleic acids: |
| Alanine (dp) | Arginine (dp) | Asparagine (dp) | Aspartic acid (dp) | Cysteine (dp) | Glutamic acid (dp) | Glutamine (dp) | Glycine (dp) | Histidine (dp) | Isoleucine (dp) | Leucine (dp) | Lysine (dp) | Methionine (dp) | Phenylalanine (dp) | Proline (dp) | Serine (dp) | Threonine (dp) | Tryptophan (dp) | Tyrosine (dp) | Valine (dp) | ||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.