Lycopene
| Lycopene | |
|---|---|
| Lycopene | |
| General | |
| Systematic name | ? |
| Other names | ? |
| Molecular formula | ? |
| SMILES | ? |
| Molar mass | ?.?? g/mol |
| Appearance | ? |
| CAS number | [?-?-?] |
| Properties | |
| Density and phase | ? g/cm³, ? |
| Solubility in water | ? g/100 ml (?°C) |
| Melting point | ?°C (? K) |
| Boiling point | ?°C (? K) |
| Acidity (pKa) | ? |
| Basicity (pKb) | ? |
| Chiral rotation [α]D | ?° |
| Viscosity | ? cP at ?°C |
| Structure | |
| Molecular shape | ? |
| Coordination geometry |
? |
| Crystal structure | ? |
| Dipole moment | ? D |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | ? |
| NFPA 704 | |
| Flash point | ?°C |
| R/S statement | R: ? S: ? |
| RTECS number | ? |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Other anions | ? |
| Other cations | ? |
| Related ? | ? |
| Related compounds | ? |
| Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Lycopene is a bright red carotenoid pigment and phytochemical found in tomatoes and other red fruits. Its name is derived from the tomato's species classification, Solanum lycopersicum where "lyco" is Greek for wolf, "persicum" means peach and tomato implies "wolf-peach".
In plants, algae, and other photosynthetic organisms, lycopene is an important intermediate in the biosynthesis of many carotenoids, including beta carotene, responsible for yellow, orange or red pigmentation, photosynthesis, and photo-protection. Structurally, it is a tetraterpene assembled from eight isoprene units, composed entirely of carbon and hydrogen, and is insoluble in water. Lycopene's eleven conjugated double bonds give it its deep red color and are responsible for its antioxidant activity. Due to its strong color and non-toxicity, lycopene is a useful food coloring.
Lycopene is not an essential nutrient for humans, but is commonly found in the diet, mainly from dishes prepared with tomato sauce. When absorbed from the stomach, lycopene is transported in the blood by various lipoproteins and accumulates in the liver, adrenal glands, and testes.
Because preliminary research has shown an inverse correlation between consumption of tomatoes and cancer risk, lycopene has been considered a potential agent for prevention of some types of cancers, particularly prostate cancer. However, this area of research and the relationship with prostate cancer have been deemed insufficient of evidence for health claim approval by the US Food and Drug Administration (see below under Antioxidant properties and potential health benefits).
Structure and physical properties
Lycopene is a symmetrical tetraterpene assembled from 8 isoprene units. It is a member of the carotenoid family of compounds, and because it consists entirely of carbon and hydrogen, is also a carotene.[1] Isolation procedures for lycopene were first reported in 1910, and the structure of the molecule was determined by 1931. In its natural, all-trans form, the molecule is long and straight, constrained by its system of eleven conjugated double bonds. Each double bond in this extended π electron system reduces the energy required for electrons to transition to higher energy states, allowing the molecule to absorb visible light of progressively longer wavelengths. Lycopene absorbs all but the longest wavelengths of visible light, so it appears red.[2]
Plants and photosynthetic bacteria naturally produce all-trans lycopene, but a total of 72 geometric isomers of the molecule are sterically possible.[3] When exposed to light or heat, lycopene can undergo isomerization to any of a number of these cis-isomers, which have a bent rather than linear shape. Different isomeres were shown to have different stabilities due to their molecular energy (highest stability: 5-cis ≥ all-trans ≥ 9-cis ≥ 13-cis > 15-cis > 7-cis > 11-cis: lowest).[4] In the human bloodstream, various cis-isomers constitute more than 60% of the total lycopene concentration, but the biological effects of individual isomers have not been investigated.[5]
Lycopene is insoluble in water, and can be dissolved only in organic solvents and oils. Because of its non-polarity, lycopene in food preparations will stain any sufficiently porous material, including most plastics. While a tomato stain can be fairly easily removed from fabric (provided the stain is fresh), lycopene diffuses into plastic, making it impossible to remove with hot water or detergent. If lycopene is oxidized (for example, by reacting with bleaches or acids), the double bonds between the carbon atoms will be broken; cleaving the molecule, breaking the conjugated double bond system, and eliminating the chromophore.
Role in photosynthesis
Carotenoids like lycopene are important pigments found in photosynthetic pigment-protein complexes in plants, photosynthetic bacteria, fungi, and algae. They are responsible for the bright colors of fruits and vegetables, perform various functions in photosynthesis, and protect photosynthetic organisms from excessive light damage. Lycopene is a key intermediate in the biosynthesis of many important carotenoids, such as beta-carotene, and xanthophylls.
Biosynthesis
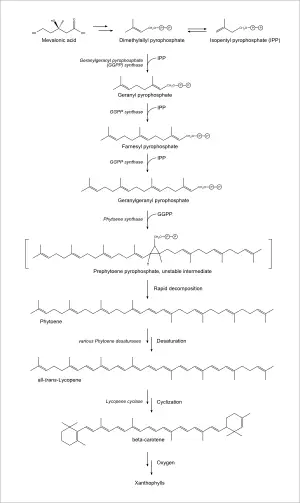
The biosynthesis of lycopene in eukaryotic plants and in prokaryotic cyanobacteria is similar, as are the enzymes involved.[6] Synthesis begins with mevalonic acid, which is converted into dimethylallyl pyrophosphate. This is then condensed with three molecules of isopentenyl pyrophosphate (an isomer of dimethylallyl pyrophosphate), to give the twenty carbon geranylgeranyl pyrophosphate. Two molecules of this product are then condensed in a tail-to-tail configuration to give the forty carbon phytoene, the first committed step in carotenoid biosynthesis. Through several desaturation steps, phytoene is converted into lycopene. The two terminal isoprene groups of lycopene can be cyclized to produce beta carotene, which can then be transformed into a wide variety of xanthophylls.[7]
Dietary sources
| Dietary sources of lycopene[8] | |
|---|---|
| Source | μg/g wet weight |
| Gac | 2,000–2,300 |
| Raw tomato | 8.8–42 |
| Tomato juice | 86–100 |
| Tomato sauce | 63–131 |
| Tomato ketchup | 124 |
| Watermelon | 23–72 |
| Pink grapefruit | 3.6–34 |
| Pink guava | 54 |
| Papaya | 20–53 |
| Rosehip puree | 7.8 |
| Apricot | < 0.1 |
Fruits and vegetables that are high in lycopene include gac, tomatoes, watermelon, pink grapefruit, pink guava, papaya, red bell pepper, seabuckthorn, wolfberry (goji, a berry relative of tomato), and rosehip. Although gac (Momordica cochinchinensis Spreng) has the highest content of lycopene of any known fruit or vegetable, up to 70 times more than tomatoes for example[9], due to gac's rarity outside its native region of SE Asia, tomatoes and tomato based sauces, juices, and ketchup account for more than 85% of the dietary intake of lycopene for most people.[10] The lycopene content of tomatoes depends on species and increases as the fruit ripens.[11]
Unlike other fruits and vegetables, where nutritional content such as vitamin C is diminished upon cooking, processing of tomatoes increases the concentration of bioavailable lycopene. Lycopene in tomato paste is four times more bioavailable than in fresh tomatoes.
While most green leafy vegetables and other sources of lycopene are low in fats and oils, lycopene is insoluble in water and is tightly bound to vegetable fiber. Processed tomato products such as pasteurized tomato juice, soup, sauce, and ketchup contain the highest concentrations of bioavailable lycopene from tomato based sources.
Cooking and crushing tomatoes (as in the canning process) and serving in oil-rich dishes (such as spaghetti sauce or pizza) greatly increases assimilation from the digestive tract into the bloodstream. Lycopene is fat-soluble, so the oil is said to help absorption. Gac is a notable exception containing high concentrations of lycopene and also saturated and unsaturated fatty acids.[citation needed]
Lycopene may be obtained from vegetables and fruits such as the tomato, but another source of lycopene is the fungus Blakeslea trispora. Gac is a promising commercial source of lycopene for the purposes of extraction and purification.
Pharmacokinetics
| Distribution of lycopene[12] | |
|---|---|
| Tissue | nmol/g wet weight |
| Liver | 1.28–5.72 |
| Kidney | 0.15–0.62 |
| Adrenal | 1.9–21.6 |
| Testes | 4.34–21.4 |
| Ovary | 0.25–0.28 |
| Adipose | 0.2–1.3 |
| Lung | 0.22–0.57 |
| Colon | 0.31 |
| Breast | 0.78 |
| Skin | 0.42 |
After ingestion, lycopene is incorporated into lipid micelles in the small intestine. These micelles are formed from dietary fats and bile acids, and help to solubilize the hydrophobic lycopene and allow it to permeate the intestinal mucosal cells by a passive transport mechanism. Little is known about the liver metabolism of lycopene, but like other carotenoids, lycopene is incorporated into chylomicrons and released into the lymphatic system. In blood plasma, lycopene is eventually distributed into the very low and low density lipoprotein fractions.[13] Lycopene is mainly distributed to fatty tissues and organs such as the adrenal glands, liver, and testes.
Adverse effects
Lycopene is non-toxic and is commonly found in the diet, but cases of excessive carotenoid intake have been reported. In a middle aged woman who had prolonged and excessive consumption of tomato juice, her skin and liver were colored orange-yellow and had elevated levels of lycopene in her blood. After three weeks on a lycopene-free diet her skin color returned to normal.[13]
Antioxidant properties and potential health benefits
Lycopene may be the most powerful carotenoid quencher of singlet oxygen[14], being 100 times more efficient in test tube studies of singlet-oxygen quenching action than vitamin E, which in turn has 125 times the quenching action of glutathione (water soluble)[citation needed]. Singlet oxygen produced during exposure to ultraviolet light is a primary cause of skin aging.[15]
Given its antioxidant properties, substantial scientific and clinical research has been devoted to a possible correlation between lycopene consumption and general health. Early research suggested some amelioration { combating of } of cardiovascular disease, cancer, diabetes, osteoporosis, and even male infertility.[16]
After extensive review reported in November 2005, the United States Food and Drug Administration has cast significant doubt on the potential for lowering disease risk, showing no link between lycopene and prevention of prostate cancer.[17] The FDA review permitted a highly limited qualified claim to be used for tomatoes and tomato products which contain lycopene, as a guide that would not mislead consumers, namely:
Very limited and preliminary scientific research suggests that eating one-half to one cup of tomatoes and/or tomato sauce a week may reduce the risk of prostate cancer. FDA concludes that there is little scientific evidence supporting this claim.
The related carotenoid antioxidant, beta-carotene, has been shown to increase the number of prostate cancer cases in a subset of patients,[18] although this area of research remains controversial and ongoing.
See also
Notes and references
Notes
- ↑ Grossman et al. (2004) p. 129
- ↑ Rao et al. (2007) p. 210
- ↑ 1054 isomers are theoretically possible, but only 72 are possible due to steric hinderance. IARC Handbook, (1998) p. 25
- ↑ Chasse et al. Journal of Molecular Structure: THEOCHEM, Volume 571, Number 1, 27 August 2001 , pp. 27-37(11)[1]
- ↑ Lycopene: Its role in human health and disease, Rao 'et al.', AGROFood industry hi-tech, July/August 2003[2]
- ↑ Cunningham (2007) p. 533
- ↑ Armstrong (1996) p. 229
- ↑ Rao and Rao (2007) pp. 209–210
- ↑ USDA study on Cartenoid content of gac fruit
- ↑ Rao (2007) p.
- ↑ Khan et al. (2008) p. 495
- ↑ Stahl (1996) p. 7
- ↑ 13.0 13.1 Stahl (1996) p. 6
- ↑ Di Mascio (1989) pp. 532–538
- ↑ Berneburg (1999) pp. 15345–15349
- ↑ Giovannucci (1995) pp. 1767–76
- ↑ Qualified Health Claims: Letter Regarding Tomatoes and Prostate Cancer(Lycopene Heath Claim Coalition)(Docket No. 2004Q-0201) US FDA/CFSAN November 2005[3]
- ↑ American Association for Cancer Research, , "No Magic Tomato? Study Breaks Link between Lycopene and Prostate Cancer Prevention", Science Daily, May 17, 2007.
ReferencesISBN links support NWE through referral fees
- Armstrong GA, Hearst JE (1996). Carotenoids 2: Genetics and molecular biology of carotenoid pigment biosynthesis. FASEB J. 10 (2): 228–37.
- Basu A, Imrhan V (2007). Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur J Clin Nutr 61 (3): 295–303.
- Berneburg M, Grether-Beck S, Kurten V, Ruzicka T, Briviba K, Sies H, Krutmann J (1999). Singlet oxygen mediates the UVA-induced generation of the photoaging-associated mitochondrial common deletion. The Journal of Biological Chemistry 274 (22): 15345–15349.
- Britton, George; Synnove Liaaen-Jensen; Hanspeter Pfander; (1996). Carotenoids : Synthesis (Carotenoids). Boston: Birkhauser. ISBN 3-7643-5297-3.
- Cunningham FX, Lee H, Gantt E (2007). Carotenoid biosynthesis in the primitive red alga Cyanidioschyzon merolae. Eukaryotic Cell 6 (3): 533–45.
- Di Mascio P, Kaiser S, Sies H (1989). Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 274 (2): 532–8.
- Gerster H (1997). The potential role of lycopene for human health. J Am Coll Nutr 16 (2): 109–26.
- Giovannucci E, Ascherio A, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1995). Intake of carotenoids and retinol in relation to risk of prostate cancer. J. Natl. Cancer Inst. 87 (23): 1767–76.
- Grossman AR, Lohr M, Im CS (2004). Chlamydomonas reinhardtii in the landscape of pigments. Annu. Rev. Genet. 38: 119–73.
- IARC Working Group on the Evaluation of Cancer Preventive Agents (1998). IARC Handbooks of Cancer Prevention: Volume 2: Carotenoids (IARC Handbooks of Cancer Prevention). Oxford University Press, USA, 25. ISBN 92-832-3002-7.
- Khan N, Afaq F, Mukhtar H (2008). Cancer chemoprevention through dietary antioxidants: progress and promise. Antioxid. Redox Signal. 10 (3): 475–510.
- Rao AV, Rao LG (March 2007). Carotenoids and human health. Pharmacol. Res. 55 (3): 207–16.
- Stahl W, Sies H (1996). Lycopene: a biologically important carotenoid for humans?. Arch. Biochem. Biophys. 336 (1): 1–9.
External links
- Phytochemicals as Nutraceuticals-Lycopene
- lycopene.org - A website promoting a lycopene rich diet.
- USDA Webpage on Lycopene Content of Gac - Fatty Acids and Carotenoids in Gac (Momordica Cochinchinensis Spreng) Fruit.
| ||||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.