Fullerene
The fullerenes are recently-discovered allotropes of carbon. They are molecules composed entirely of carbon, which take the form of a hollow sphere, ellipsoid, or tube. Spherical fullerenes are sometimes called buckyballs, while cylindrical fullerenes are called buckytubes or nanotubes.
Naming
Buckminsterfullerene (C60) was named for Richard Buckminster Fuller, a noted architect who popularized the geodesic dome. Since buckminsterfullerenes have a similar shape to that sort of dome, the name was thought to be appropriate.
Fullerenes are similar in structure to graphite, which is composed of a sheet of linked hexagonal rings, but they contain pentagonal (or sometimes heptagonal) rings that prevent the sheet from being planar.
Buckminsterfullerene
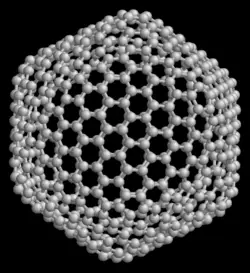
The smallest fullerene in which no two pentagons share an edge (which is destabilizing — see pentalene) is C60 (buckminsterfullerene), and this is also the most common.
The structure of C60 is that of a truncated icosahedron, which resembles a round soccer ball of the type made of hexagons and pentagons, with a carbon atom at the corners of each hexagon and a bond along each edge. A polymerized single-walled nanotubule (P-SWNT) is a substance composed of polymerized fullerenes in which carbon atoms from one buckytube bond with carbons in other buckytubes.
Prediction and discovery
In molecular beam experiments, discrete peaks were observed corresponding to molecules with the exact mass of 60, 70, or greater numbers of carbon atoms. Harold Kroto, from the University of Sussex, James Heath, Sean O'Brien, Robert Curl and Richard Smalley, from Rice University, discovered C60 and the fullerenes in 1985. Kroto, Curl, and Smalley were awarded the 1996 Nobel Prize in Chemistry for their roles in the discovery of this class of compounds. C60 and other fullerenes were later noticed occurring outside of a laboratory environment (e.g. in normal candle soot). By 1991, it was relatively easy to produce grams of fullerene powder using the techniques of Donald Huffman and Wolfgang Krätschmer. Fullerene purification remains a challenge to chemists and determines fullerene prices to a large extent. So called endohedral fullerenes have ions or small molecules incorporated inside the cage atoms. Fullerene is an unusual reactant in many organic reactions such as the Bingel reaction discovered in 1993.
Properties
As of the early twenty-first century, the chemical and physical properties of fullerenes are still under heavy study, in both pure and applied research labs. In April 2003, fullerenes were under study for potential medicinal use — binding specific antibiotics to the structure to target resistant bacteria and even target certain cancer cells such as melanoma. In the October 2005 issue of Chemistry and Biology, an article [1] describing the use of fullerenes as light-activated antimicrobial agents was published.
Fullerenes are not very reactive due to the stability of the graphite-like bonds, and are also sparingly soluble in many solvents. Common solvents for the fullerenes include toluene and carbon disulfide. Solutions of pure Buckminsterfullerene have a deep purple color. Fullerenes are the only known allotrope of carbon that can be dissolved. Researchers have been able to increase the reactivity by attaching active groups to the surfaces of fullerenes. Buckminsterfullerene does not exhibit "superaromaticity". That is, the electrons in the hexagonal rings do not delocalize over the whole molecule.
Other atoms can be trapped inside fullerenes, and indeed recent evidence for a meteor impact at the end of the Permian period was found by analysing noble gases so preserved.
In the field of nanotechnology, heat resistance and superconductivity are some of the more heavily studied properties.
A common method used to produce fullerenes is to send a large current between two nearby graphite electrodes in an inert atmosphere. The resulting carbon plasma arc between the electrodes cools into sooty residue from which many fullerenes can be isolated.
Possible dangers
Although buckyballs have been thought in theory to be relatively inert, a presentation given to the American Chemical Society in March 2004 and described in an article in New Scientist on April 3 2004, suggests the molecule is injurious to organisms. An experiment by Eva Oberdörster at Southern Methodist University, which introduced fullerenes into water at concentrations of 0.5 parts per million, found that largemouth bass suffered a 17-fold increase in cellular damage in the brain tissue after 48 hours. The damage was of the type lipid peroxidation, which is known to impair the functioning of cell membranes. There were also inflammatory changes in the liver and activation of genes related to the making of repair enzymes. At the time of presentation, the SMU work had not been peer reviewed.
Because of their notable properties, buckyballs may be part of many products in the near future. With this knowledge in mind, many researchers are investigating ways to reduce the toxicity of the buckyballs [2]. They have discovered that they can reduce the toxicity by adding hydroxyls, among other chemical groups. With each chemical group added to the buckyball, the scientists could reduce its toxicity level by an order of magnitude.
It is believed that buckyballs acquire their toxicity by producing free radicals in water that damage the lipids on the cellular membranes of animals, destroying the cells.
Pristine C60 can be suspended in water at low concentrations as large clusters often termed nC60. These clusters are spherical clumps of C60 between 250-350 nm in diameter. Thus, nC60 represents a different chemical entity than solutions of C60 in which the fullerenes exist as individual molecules. Recently, results presented at the ACS meeting in Anaheim, CA suggest that nC60 is moderately toxic to water fleas and juvenile largemouth bass at concentrations in water of around 800 ppb. The first study of its kind on marine life, these preliminary results quickly spread across the scientific community. However, the overwhelming evidence of the essential non-toxicity of C60 (not nC60) in previously peer-reviewed articles of C60 and many of its derivatives indicates that our compounds are likely to have little (if any) toxicity, especially at the very low concentration at which it is used (~1-10 µM).
Fullerene extract mixture (C60/C70) solubility
Solvents that dissolve fullerenes are listed below in order from highest solubility. The value in parentheses is the approximate saturated concentration.
- 1,2,4-trichlorobenzene (20mg/ml)
- carbon disulfide (12mg/ml)
- toluene (3.2mg/ml)
- benzene (1.8mg/ml)
- chloroform (0.5mg/ml)
- carbon tetrachloride (0.4mg/ml)
- cyclohexane (0.054mg/ml)
- n-hexane (0.046mg/ml)
- THF (0.037mg/ml)
- acetonitrile (0.02mg/ml)
- methanol (0.0009mg/ml)
Diffraction of fullerene
In 1999, researchers from the University of Vienna Template:An demonstrated that the wave-particle duality applied to macro-molecules such as fullerene.
Notes
- Template:Anb Wave-particle duality of C60, M. Arndt , O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, A. Zeilinger, Nature 401, 680-682, 14 October 1999
Mathematics of fullerenes
In mathematical terms, the structure of a fullerene is a trivalent convex polyhedron with pentagonal and hexagonal faces. Using Euler formula F - E + V = 2, (plus the fact that every vertex in a fullerene structure belongs to exactly 3 faces) one can easily prove that there are exactly 12 pentagons in a fullerene. The smallest fullerene is C20, the dodecahedron. There are no fullerenes with 22 vertices. The number of fullerenes C2n grows rapidly with increasing n = 12,13, ... For instance, there are 1812 non-isomorphic fullerenes C60 but only one of them, the buckminsterfullerene alias truncated icosahedron, has no pair of adjacent pentagons.
Carbon nanotube
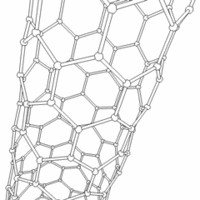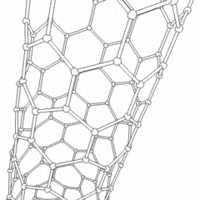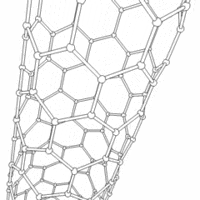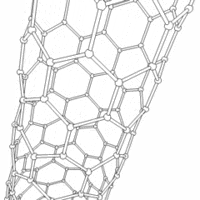
Carbon nanotubes are cylindrical carbon molecules with novel properties that make them potentially useful in a wide variety of applications (e.g., nano-electronics, optics, materials applications, etc.). They exhibit extraordinary strength and unique electrical properties, and are efficient conductors of heat. Inorganic nanotubes have also been synthesized.
A nanotube (also known as a buckytube) is a member of the fullerene structural family, which also includes buckyballs. Whereas buckyballs are spherical in shape, a nanotube is cylindrical, with at least one end typically capped with a hemisphere of the buckyball structure. Their name is derived from their size, since the diameter of a nanotube is on the order of a few nanometers (approximately 50,000 times smaller than the width of a human hair), while they can be up to several centimeters in length. There are two main types of nanotubes: single-walled nanotubes (SWNTs) and multi-walled nanotubes (MWNTs).
Nanotubes are composed entirely of sp² bonds, similar to graphite. Stronger than the sp³ bonds found in diamond, this bonding structure provides them with their unique strength. Nanotubes naturally align themselves into "ropes" held together by Van der Waals forces. Under high pressure, nanotubes can merge together, trading some sp² bonds for sp³ bonds, giving great possibility for producing strong, unlimited-length wires through high-pressure nanotube linking. [3]
While it has long been known that carbon fibers can be produced with a carbon arc, and patents were issued for the process, it was not until 1991 that Sumio Iijima, a researcher with the NEC Laboratory in Tsukuba, Japan, observed that these fibers were hollow. This feature of nanotubes is of great interest to physicists because it permits experiments in one-dimensional quantum physics.
Single-walled nanotubes
Most SWNTs have a diameter of close to 1nm, with a tube length that can be many thousands of times larger. The structure of a SWNT can be conceptualized by wrapping a one-atom-thick layer of graphite (called graphene) into a seamless cylinder. The way the graphene sheet is wrapped is represented by a pair of indices (n,m) called the chiral vector. The integers n and m denote the number of unit vectors along two directions in the honeycomb lattice of graphene. If m=0, the nanotubes are called "zigzag". If n=m, the nanotubes are called "armchair". Otherwise, they are called "chiral".
SWNTs are a very important variety of carbon nanotube because they exhibit important electric properties that are not shared by the multi-walled carbon nanotube (MWNT) variants. SWNTs are the most likely candidate for miniaturizing electronics past the microelectromechanical scale that is currently the basis of modern electronics. The most basic building block of these systems is the electric wire, and SWNTs can be excellent conductors. (Dekker, et al., 1999) One useful application of SWNTs is in the development of the first intramolecular field effect transistors (FETs). The production of the first intramolecular logic gate useing SWNT FETs has recently become possible as well (Derycke, et al., 2001). To create a logic gate you must have both a p-FET and an n-FET. Because SWNTs are p-FETs when exposed to air and n-FETs when unexposed to oxygen, they were able to protect half of a SWNT from oxygen exposure, while exposing the other half to oxygen. The result was a single SWNT that acted as a NOT logic gate with both p and n-type FETs within the same molecule.
SWNTs are still very expensive to produce, and the development of more affordable synthesis techniques is vital to the future of carbon nanotechnology. If cheaper means of synthesis cannot be discovered, it would make it financially impossible to apply this technology to commercial-scale applications.
Properties
The covalent bonding undergone in CNTs means they have very high tensile strengths. In 2000, a SWNT was tested to have a tensile strength of 63 GPa. In comparison, high-carbon steel has a tensile strength of approximately 1.2 GPa. CNTs also have very high elastic modulus, in the order of 1 TPa [4].
Under excessive tensile strain, the tubes will undergo plastic deformation, which means the deformation is permanent. This deformation begins at strains of approximately 5% [Qian et al, 2002] and can increase the maximum strain the tube undergoes before fracture by releasing strain energy.
CNTs are not nearly as strong under compression. Due to their hollow structure, they tend to undergo buckling, when placed under compressive, torsional or bending stress.
Due to the symmetry and unique electronic structure of graphene, the structure of a nanotube strongly affects its electrical properties. For a given (n,m) nanotube, if 2n + m=3q (where q is an integer), then the nanotube is metallic, otherwise the nanotube is a semiconductor. Thus all armchair (n=m) nanotubes are metallic, and nanotubes (5,0), (6,4), (9,1), etc. are semiconducting. An alternative (equivalent) representation of this condition is if (n - m) /3=integer, then the SWNT is metallic. In theory, metallic nanotubes can have an electrical current density more than 1,000 times stronger than metals such as silver and copper.
All nanotubes are expected to be very good thermal conductors along the tube, exhibiting a property known as "ballistic conduction," but good insulators laterally to the tube axis.
As with any material, the existence of defects affect the material properties. Defects can occur in the form of atomic vacancies. High levels of such defects can lower the tensile strength by up to 85% [5]. Due to the almost one-dimensional structure of CNTs, the tensile strength of the tube is dependent on the weakest segment of it in a similar manner to a chain, where a defect in a single link will greatly diminish the strength of the entire chain.
In terms of the tube's electrical properties, they too are affected by the presence of defects. A common result is the lowered conductivity through the defected region of the tube. Some defect formation in armchair type tubes (which are metallic) can cause the region surrounding that defect to become semiconducting.
Synthesis
Techniques have been developed to produce nanotubes in sizeable quantities, but their cost still prohibits any large scale use of them. Fullerenes and carbon nanotubes are not necessarily products of high-tech laboratories, they are commonly formed in such mundane places as candle flames. However, these naturally occurring varieties are highly irregular in size and quality, and the high degree of uniformity necessary to meet the needs of research and industry is impossible in such an uncontrolled environment. There are several methods employed to make nanotubes, such as arc discharge, laser ablation, high pressure carbon monoxide (HiPco), and chemical vapor deposition (CVD). In general, the CVD method has shown the most promise in being able to produce larger quantities of nanotube (compared to the other methods) at lower cost. This is usually done by reacting a carbon-containing gas (such as acetylene, ethylene, ethanol, etc.) with a metal catalyst particle (usually cobalt, nickel, iron or a combination of these as cobalt/iron or cobalt/molybdenium) at temperatures above 600°C.
Applications
The strength and flexibility of carbon nanotubes makes them of potential use in controlling other nanoscale structures, which suggests they will have an important role in nanotechnology engineering. The highest tensile strength an individual SWNT has been tested to be is 63 GPa [6]. In Earth's upper atmosphere, atomic oxygen erodes the carbon nanotubes, but other applications of carbon nanotubes rarely need the surface to be protected. Though it is debatable if nanotube materials can ever be made with a tensile strength approaching that of individual tubes, composites may still yield incredible strengths potentially sufficient to allow the building of such things as space elevators, artificial muscles, ultrahigh-speed flywheels, and more. MIT is working on combat jackets utilizing carbon nanotubes for ultrastrong fibers and for monitoring its wearer's condition.
Carbon nanotubes have already been used as composite fibers in polymers and concrete to improve the mechanical, thermal and electrical properties of the bulk product. Researchers have also found that adding them to polyethylene increases the polymer's elastic modulus by 30%. In concrete, they increase the tensile strength, and halt crack propagation.
Conductive carbon nanotubes have been used for several years in brushes for commercial electric motors. They replace traditional carbon black, which is mostly impure spherical carbon fullerenes. The nanotubes improve electrical and thermal conductivity because they stretch through the plastic matrix of the brush. This permits the carbon filler to be reduced from 30% down to 3.6%, so that more matrix is present in the brush. Nanotube composite motor brushes are better-lubricated (from the matrix), cooler-running (both from better lubrication and superior thermal conductivity), less brittle (more matrix, and fiber reinforcement), stronger and more accurately moldable (more matrix). Since brushes are a critical failure point in electric motors, and also don't need much material, they became economical before almost any other application.
A recent 2005 Science paper notes that drawing transparent high strength swathes of SWNT is a functional production technique. These conductive elastic materials are among the many applications listed here of photovoltaic active structures as well as load structures.
Carbon nanotubes additionally can also be used to produce nanowires of other chemicals, such as gold or zinc oxide. These nanowires in turn can be used to cast nanotubes of other chemicals, such as gallium nitride. These can have very different properties from CNTs - for example, gallium nitride nanotubes are hydrophilic, while CNTs are hydrophobic, giving them possible uses in organic chemistry that CNTs could not be used for.
One use for nanotubes that has already been developed is as extremely fine electron guns, which could be used as miniature cathode ray tubes in thin high-brightness low-energy low-weight displays. This type of display would consist of a group of many tiny CRTs, each providing the electrons to hit the phosphor of one pixel, instead of having one giant CRT whose electrons are aimed using electric and magnetic fields. These displays are known as field emission displays (FEDs). A nanotube formed by joining nanotubes of two different diameters end to end can act as a diode, suggesting the possibility of constructing electronic computer circuits entirely out of nanotubes. Nanotubes have been shown to be superconducting at low temperatures.
Nanotubes can be opened and filled with materials such as biological molecules, raising the possibility of applications in biotechnology. They can be used to dissipate heat from tiny computer chips.
Carbon nanotube fiber & film
One application for nanotubes that is currently being researched is high tensile strength fibers. Two methods are currently being tested for the manufacture of such fibers. A French team has developed a liquid spun system that involves pulling a fiber of nanotubes from a bath which yields a product that is approximately 60% nanotubes. The other method, which is simpler but produces weaker fibers uses traditional melt-drawn polymer fiber techniques with nanotubes mixed in the polymer. After drawing, the fibers can have the polymer burned out of them to make them purely nanotube or they can be left as they are.
Ray Baughman's group from the NanoTech Institute at University of Texas at Dallas produced the current toughest material known in mid-2003 by spinning fibers of single wall carbon nanotubes with polyvinyl alcohol. Beating the previous contender, spider silk, by a factor of four, the fibers require 600 J/g to break. In comparison, the bullet-resistant fiber Kevlar is 27-33J/g. In mid-2005 Baughman and co-workers from Australia's Commonwealth Scientific and Industrial Research Organization developed a method for producing transparent carbon nanotube sheets 1/1000th the thickness of a human hair capable of supporting 50,000 times their own mass. In August 2005, Ray Baughman's team managed to develop a fast method to manufacture up to seven meters per minute of nanotube tape [7]. Once washed with ethanol, the ribbon is only 50 nanometers thick; a square kilometer of the material would only weigh 30 kilograms.
In 2004 Alan Windle's group of scientists at the Cambridge-MIT Institute developed a way to make carbon nanotube fiber continuously at the speed of several centimetres per second just as nanotubes are produced. One thread of carbon nanotubes was more than 100 metres long. The resulting fibers are electrically conductive and as strong as ordinary textile threads. [8] [9]
Current progress
- In April of 2001, IBM announced it had developed a technique for automatically developing pure semiconductor surfaces from nanotubes.
- On September 19, 2003, NEC Corporation, Japan, announced stable fabrication technology of carbon nanotube transistors.
- High purity (80%) nanotubes were reported in June 2003 with metallic properties can be extracted with electrophoretic techniques. [10]
- As of 2003, nanotubes cost from 20 euro per gram to 1000 euro per gram, depending on purity, composition (single-wall, double-wall, multi-wall) and other characteristics.
- In June 2004 scientists from China's Tsinghua University and Louisiana State University demonstrated the use of nanotubes in incandescent lamps, replacing a tungsten filament in a lightbulb with a carbon nanotube one.
- In 2004, Nature published a photo of an individual 4 cm long single-wall nanotube (SWNT).
- In August 2005, GE announced the development of an ideal carbon nanotube diode that operates at the "theoretical limit," or best possible performance. The company also observed a photovoltaic effect in the nanotube diode device that could lead to breakthroughs in solar cells that make them more efficient and a more viable alternative in the mainstream energy market.[11]
- In September of 2005 Texas-based Applied Nanotech, in conjunction with six Japanese electronics firms, have created a prototype of a 25-inch TV using carbon nanotubes. The prototype TV does not suffer from "ghosting," as some types of digital TVs.
- In September 2005 researchers at Lawrence Livermore National Laboratory demonstrated that ignition by a conventional flashbulb takes place when a layer of 29% iron enriched SWNT is placed on top of a layer of explosive material such as PETN. With ordinary explosives optical ignition is only possible with high powered lasers [12].
- In September 2005 researchers demonstrated a new way to coat MWNT's with magnetite which after orientation in a magnetic field were able to attract each other over a distance of at least 10 micrometres. [13]. The nanotubes were functionalized with negatively charged carboxylic acid groups in a AIBN type free radical addition. Magnetite nanoparticles prepared by the Massart method were given a positive charge by washing with nitric acid which made them stick to the nanotubes by electrostatic forces.
- In September 2005, Korean scientists lead by Pohang University of Science and Technology Professor Kim Kwang-Soo developed the longest carbon-nanotubes; they succceeded in pulling out the nested tubes from the multiwalled nanotubes (MWNTs). They extracted 1 millimeter, 1,000 times longer than the previous achievement of 1 micrometer.
Carbon nanotubes in electrical circuits
Carbon nanotubes have many properties—from their unique dimensions to an unusual current conduction mechanism—that make them ideal components of electrical circuits. Currently, there is no reliable way to arrange carbon nanotubes into a circuit.
The major hurdles that must be jumped for carbon nanotubes to find prominent places in circuits relate to fabrication difficulties. The carbon nanotube production processes are very different from the traditional IC fabrication process. The IC fabrication process is somewhat like sculpture—films are deposited onto a wafer and pattern-etched away. Carbon nanotubes are fundamentally different from films; they are like atomic-level spaghetti (and every bit as sticky).
Researchers sometimes resort to manipulating nanotubes one-by-one with the tip of an atomic force microscope in a painstaking, time-consuming process. Perhaps the best hope is that carbon nanotubes can be grown through a chemical vapor deposition process from patterned catalyst material on a wafer, which serve as growth sites and allow designers to position one end of the nanotube. During the deposition process, an electric field can be applied to direct the growth of the nanotubes, which tend to grow along the field lines from negative to positive polarity. Another way for the self assembly of the carbon nanotube transistors consist in using chemical or biological techniques to place the nanotubes from solution to determinate place on a substrate.
Even if nanotubes could be precisely positioned, there remains the problem that, to this date, engineers have been unable to control the types of nanotubes—metallic, semiconducting, single-walled, multi-walled—produced. A chemical engineers solution is needed if nanotubes are to become feasible for commercial circuits.
External links and sources
- The Nanotube site - This site last update: 2005.08.05 (Friday) 10:08:05 EDT by David Tomanek.
- "TubeGen Online: Web-Accessible Nanotube Structure Generator"
- Industrial source for MWNT CNT manufacturer in Japan
- Multi-Wall-Nanotubes, Nanofibers, metallised Nanotubes manufacturer in Germany
- Commercial source of carbon nanotubes NTP-nanotube manufacturer in China
- Ahwahnee Technology Silicon Valley carbon nanotube developer
- The wonderous World of Carbon Nanotubes (Good introduction to nanotubes)
- Jamieson V. "Open secret" New Scientist
- Nantero (developers of nanotube based non-volatile memory)
- University of Cambridge, UK, Research group website (Affordable methods for making carbon nanotubes and using them for gene delivery)
- University of Texas at Dallas NanoTech Institute
- NanoDiamond (nanotubes arranged in a diamond formation yielding a very high strength-to-weight ratio material)
- Carbon Nanotube & Fullerene Models - Vincent Herr, Houston, TX
- Science News - Nanotube Super Fibers - From Science News, Vol. 163, No. 24, June 14, 2003, p. 372. No Updates.
- Nanotube production surveys Last Update September 18, 2005
- Carbon Nanotubes Monthly Newsletter - focuses on various applications of carbon nanotubes and surveys research papers and issued patents
- Columbia University Nanoscale Science and Engineering Center presents "Our Energy Challege" September 23, 2003
- Review of Non-Oil and Gas Research Activities in the Houston-Galveston-Gulf Coast Area
- commercial sources
- Carbon Designs, Inc. Only home page. No technical data as of Sept 25, 2005.
- "For pure nanotubes add water" article by Eric Smalley 2004-12 "stands of single-wall carbon nanotubes as tall as 2.5 millimeters and 99.98 percent pure. Individual nanotubes range from one to three nanometers in diameter."
- Nanotubes show their strength in numbers (MSNBC, August 18, 2005) Super-strong sheets could be used in future screens and surfaces
- Nanotube composites, current applications and challenges, electrical conductivity records in 2005
- nanotechweb.org: news on nanotubes and other fields of nanotechnology
- Bose-Einstein Condensation of Helium and Hydrogen inside Bundles of Carbon Nanotubes
- Image of a carbon nanotube
ReferencesISBN links support NWE through referral fees
- Carbon Nanotubes and Related Structures - New Materials for the Twenty-First Century, P.J.F. Harris (Cambridge University Press, 1999) Introductory textbook
Deepak Srivastava and Chenyu Wei (2003). Nanomechanics of Carbon Nanotubes and Composite. Applied Mechanics Review 56 (2): 215–230.
Dong Qian et al. (2002). Mechanics of Carbon Nanotubes. Applied Mechanical Review 55 (6): 495–533.
Dekker, C., Carbon Nanotubes as Molecular Quantum Wires, Phys. Today, 1999, May, 22-28.
Derycke, V., Mertel, R., Appenzeller, J., Avouris, Ph., Carbon Nanotube Inter- and Intramolecular Logic Gates, Nano Lett., 2001, 1, 453-456.
da:Kulstof-nanorør de:Kohlenstoffnanoröhre es:Nanotubo fi:Nanoputki fr:Nanotube it:Nanotubo di carbonio ja:カーボンナノチューブ pl:Nanorurka pt:Nanotubo de Carbono ru:Углеродные нанотрубки sv:Nanorör zh:碳纳米管
Media
|
|
Further reading
- The Most Beautiful Molecule: The Discovery of the Buckyball by Hugh Aldersey-Williams (John Wiley & Sons, 1995) ISBN 0-471-19333-X
See also
- Carbon nanotube
- Dodecahedrane
- Graphene
- Polyhedron
- Geodesic dome
- Prismane C8
External links
- Kim Allen
- Center for Nanoscale Science and Technology
- Dr. Smalley's brief autobiography
- Dr. Smalley's webpage
- Potential use of fullerenes in medicine
- Carbon Fullerene & Nanotube Models Vincent Herr, Houston, TX
- Fullerene Images for Web and Presentation
- Discovery of graphene
- Diffraction and Interference with Fullerenes: Wave-particle duality of C60, University of Vienna
- Fullerene-based architectures for quantum computing in Germany and in Great Britain at the QIP IRC
- Molview from bluerhinos.co.uk See Buckminsterfullerene (C60) in 3D
- Interactive 3D molecular visualization of fullerene (requires Macromedia Flash)
- Computational Chemistry Wiki
cs:Fulleren de:Fulleren es:Fulereno eo:Fulereno fr:Fullerène it:Fullerene he:פולרין nl:Fullereen ja:フラーレン pl:Fuleren pt:Buckminsterfullerenos ro:Fulerene ru:Фуллерены su:Fullerin fi:Fullereeni sv:Fulleren zh:富勒烯
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.