Difference between revisions of "Ctenophore" - New World Encyclopedia
Rick Swarts (talk | contribs) |
m (Remove * from 'optional' links) |
||
| (13 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
| − | {{ | + | {{Copyedited}}{{Paid}}{{Approved}}{{Images OK}}{{Submitted}} |
{{Taxobox_begin | color = pink | name = Comb jellies}} | {{Taxobox_begin | color = pink | name = Comb jellies}} | ||
| Line 5: | Line 5: | ||
{{Taxobox_begin_placement | color = pink}} | {{Taxobox_begin_placement | color = pink}} | ||
{{Taxobox_regnum_entry | taxon = [[Animal]]ia}} | {{Taxobox_regnum_entry | taxon = [[Animal]]ia}} | ||
| − | {{Taxobox_phylum_entry | taxon = '''Ctenophora'''}}<br/>{{Taxobox authority | author = [[Johann Friedrich von Eschscholtz|Eschscholtz]] | + | {{Taxobox_phylum_entry | taxon = '''Ctenophora'''}}<br/>{{Taxobox authority | author = [[Johann Friedrich von Eschscholtz|Eschscholtz]] | date = 1829}} |
{{Taxobox_end_placement}} | {{Taxobox_end_placement}} | ||
{{Taxobox_section_subdivision | color = pink | plural_taxon = Classes}} | {{Taxobox_section_subdivision | color = pink | plural_taxon = Classes}} | ||
| Line 12: | Line 12: | ||
{{Taxobox_end}} | {{Taxobox_end}} | ||
| − | '''Ctenophores''' (phylum '''Ctenophora'''), also known as '''comb jellies''', are marine [[invertebrate]]s that have eight rows of | + | '''Ctenophores''' (phylum '''Ctenophora'''), also known as '''comb jellies''', are marine [[invertebrate]]s that have eight rows of comb-like cilia on their transparent, gelatinous bodies. They are the largest animal to use [[cilia]] for locomotion. |
| − | Superficially, ctenophores resemble [[jellyfish]], which belong to the phylum [[Cnidaria]]. Indeed, the Ctenophores and the Cnidaria were formerly grouped together as Coelenterata. However, despite their appearance, ctenophores are zoologically not true jellyfish, not least because they lack the characteristic cnidocytes (specialized cells that carry stinging organelles) that characterize the Cnidaria. | + | Superficially, ctenophores resemble [[jellyfish]], which belong to the phylum [[Cnidaria]]. Indeed, the Ctenophores and the Cnidaria were formerly grouped together as Coelenterata. However, despite their appearance, ctenophores are zoologically not true jellyfish, not least because they lack the characteristic cnidocytes (specialized cells that carry stinging organelles) that characterize the Cnidaria. |
| − | The signature characteristic of ctenophores are the comb rows, whereby the closely-spaced cilia in each row, which are fused at the base, are arranged as a stack of combs, called comb plates or ctenes. The word ''ctenophore'' (pronounced without the ''c'') comes from [[Greek language|Greek]], ''kteno''-, ''kteis'', "comb" and -''phore'', meaning "bearer." | + | The signature characteristic of ctenophores are the comb rows, whereby the closely-spaced cilia in each row, which are fused at the base, are arranged as a stack of combs, called comb plates or ctenes. The word ''ctenophore'' (pronounced without the ''c'') comes from [[Greek language|Greek]], ''kteno''-, ''kteis'', "comb" and -''phore'', meaning "bearer." |
| − | + | As carnivores, ctenophores are integral in [[food chain|food webs]], consuming other ctenophores, small [[crustacean]]s, and other marine invertebrates, and being consumed by [[jellyfish]], sea turtles, and other ctenophores, among others. They also provide aesthetic value to [[human being|human]]s, whether through the unique light-scattering produced by the rows of cilia, which appear as a changing rainbow of colors moving down the comb rows (Mills 2005), or their gelatinous transparent and sometimes colorful (though generally colorless) bodies, or the bioluminescence of many species. However, invasive species of ctenophores have been known to severely damage [[ecosystem]]s, including a collapse of the [[Black Sea]] fisheries when an accidentally introduced ctenophore species outcompeted the [[fish]] for food. | |
| − | There are more than | + | There are more than one hundred varieties of ctenophore spread throughout the worlds' oceans, which form a considerable proportion of the entire [[plankton]] biomass. Most centophores are small (a few millimeters to several centimeters in diameter), but a few species have individuals that approach (or exceed) one meter (Mills 2005). Ctenophores live only in marine waters, and can be found from the poles to tropical zones, and from the surface to the deep ocean (Mills 2005). A few species, such as the sea gooseberry (''Pleurobrachia pileus''), native to the North Sea, have reached such high populations that they clog fishermen's nets, while of other species only a few examples are known. The fragile makeup of ctenophora makes research into their way of life extremely difficult. |
== Body == | == Body == | ||
| − | [[Image:LightRefractsOf comb-rows of ctenophore Mertensia ovum.jpg|thumb|250px|Light refracting off a Mertensia ovum | + | [[Image:LightRefractsOf comb-rows of ctenophore Mertensia ovum.jpg|thumb|250px|Light refracting off a Mertensia ovum]] |
| − | Ctenophora are often colorless, except for the coloration caused by algae cells with which they live in [[symbiosis]]. This is particularly true of ctenophores that live near the ocean surface. | + | Ctenophora are often colorless, except for the coloration caused by [[algae]] [[cell (biology)|cells]] with which they live in [[symbiosis]]. This is particularly true of ctenophores that live near the ocean surface. |
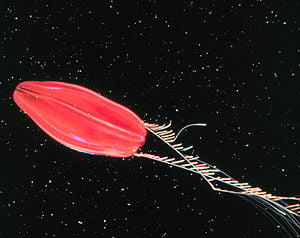
However, there are species that live in deep waters that can be highly pigmented, such as the Red Tortuga, whose scientific name is not yet established. The Red Tortuga is dark red in color and, like many other ctenophores, can give off light by means of [[bioluminescence]]. The coloration may serve as camouflage for the species that live on the sea bed. One species, ''Eurhamphaea vexilligera'', can give off a luminous red tint, which may dissuade predators. Ctenophores that live on the bottom of the ocean are often brightly colored as adults and may look like nudibranches or flatworms; some are colored similarly to their substrate (Mills 2005). | However, there are species that live in deep waters that can be highly pigmented, such as the Red Tortuga, whose scientific name is not yet established. The Red Tortuga is dark red in color and, like many other ctenophores, can give off light by means of [[bioluminescence]]. The coloration may serve as camouflage for the species that live on the sea bed. One species, ''Eurhamphaea vexilligera'', can give off a luminous red tint, which may dissuade predators. Ctenophores that live on the bottom of the ocean are often brightly colored as adults and may look like nudibranches or flatworms; some are colored similarly to their substrate (Mills 2005). | ||
| − | The beating of the eight rows of cilia can also scatter light and cause the appearance of a changing rainbow of colors moving down the comb rows (Mills 2005). This is not to be confused with bioluminescence, whose blue or | + | The beating of the eight rows of cilia can also scatter light and cause the appearance of a changing rainbow of colors moving down the comb rows (Mills 2005). This is not to be confused with bioluminescence, whose blue or green light can only be seen in darkness (Mills 2005). |
While they often are only a few centimeters long, the species of the Cestum genus can reach an average of one and a half meters. | While they often are only a few centimeters long, the species of the Cestum genus can reach an average of one and a half meters. | ||
| − | Ctenophores are considered to be "bi-radially [[symmetry (biology)|symmetrical]]", with an underlying bilaterial symmetry, as opposed to the complete [[symmetry (biology)|radial symmetry]] of the cnidarians. The main bodily axis running between the mouth and their sensory organ, the statocyst, | + | Ctenophores are considered to be "bi-radially [[symmetry (biology)|symmetrical]]", with an underlying bilaterial symmetry, as opposed to the complete [[symmetry (biology)|radial symmetry]] of the cnidarians. The main bodily axis running between the mouth and their sensory organ, the statocyst, that lies exactly opposite from the mouth, offers a radial symmetry. This symmetry is superficially broken in the lower part of the creatures by the two tentacles and in the higher part by the digestive chamber, which is separated into several channels. The lower symmetry is shifted round from the higher by ninety degrees, forming a disymmetry or a biradial form. |
| − | The body consists of two transparent cell layers, which make up its outer skin (ectoderm) and inner skin (gastroderm). The ectoderm, made up of two cell layers, is mostly covered by a protective layer of slime, excreted by special glands. The gastroderm surrounds a cavity which serves as a [[stomach]] and is only accessible by the mouth opening, connected by a long, narrow gullet. Captured quarry is pre-digested in the gullet by strong [[enzyme]]s and fully decomposed in the stomach. There is no separate exit from the stomach apart from two 'anal pores' | + | The body consists of two transparent cell layers, which make up its outer skin (ectoderm) and inner skin (gastroderm). The ectoderm, made up of two cell layers, is mostly covered by a protective layer of slime, excreted by special glands. The gastroderm surrounds a cavity which serves as a [[stomach]] and is only accessible by the mouth opening, connected by a long, narrow gullet. Captured quarry is pre-digested in the gullet by strong [[enzyme]]s and fully decomposed in the stomach. There is no separate exit from the stomach apart from two 'anal pores'—which despite their name, are not used for excretion—so indigestible waste must be expelled via the mouth. |
The space between the inner and outer skin is taken up by the mesogloea, a thick, transparent, jelly-like layer made from [[collagen]] and connective tissue, pervaded by numerous small channels, which are used for transport and storage of nutrients. The position of the channels varies from species to species, but they mostly run directly underneath the tissues that they serve. The extracellular net of structural protein is kept upright by special cells similar to amoebas. | The space between the inner and outer skin is taken up by the mesogloea, a thick, transparent, jelly-like layer made from [[collagen]] and connective tissue, pervaded by numerous small channels, which are used for transport and storage of nutrients. The position of the channels varies from species to species, but they mostly run directly underneath the tissues that they serve. The extracellular net of structural protein is kept upright by special cells similar to amoebas. | ||
| − | The mesogloea may also play a role in the lift of the creatures. [[Flagella]] found in the channels of the digestive system may serve to pump water in or out of the mesogloea, when osmotic water pressure changes, perhaps because the creature has swum out of saline sea water into coastal brackwater | + | The mesogloea may also play a role in the lift of the creatures. [[Flagella]] found in the channels of the digestive system may serve to pump water in or out of the mesogloea, when osmotic water pressure changes, perhaps because the creature has swum out of saline sea water into coastal brackwater. |
| − | + | Ctenophora do not possess a specific [[circulatory system]], neither do they have any organs for breathing; gas exchange and the excretion of waste products of cell [[metabolism]], such as [[ammonia]], occur over the body's entire surface through simple diffusion. The body is pervaded by a simple net of neurons without a "[[brain]]," with the neurons concentrated around the gullet, tentacles, "combs," and statocysts; it is connected with the muscular cells found in the mesogloea and the inner cellular layer of the ectoderm. | |
| − | + | === Statocyst, comb rows, and their use in movement=== | |
| − | + | [[Image:Ctenophore.jpg|thumb|right|300px|Red Tortuga with tentacles and clearly visible filaments]] | |
| − | + | Many ctenophora simply let themselves drift with the current. They can, however, also swim short distances by means of the strokes of their flagella and by using their mouth opening as a rudder. They are the largest animals to use their flagella for movement and can reach speeds of about five centimeters per second. A possible adaptive advantage is that constant strokes do not cause vibrations that would alert prey or predators. | |
| − | + | Some varieties also use the muscular cells of their mouth lobes to swim, while others move by undulating their body or creeping like [[flatworm]]s. | |
| − | + | The ''statocyst'' is a specialized system that serves the ctenophore as a balancing organ and also controls its movement. It can be found on the side of the body turned away from the mouth opening. It is formed by a collection of a few hundred lime cells on one side and four horizontal groups of serpentine [[flagellum|flagella]], known as the ''statolith''. As outside influences cause the ctenophore to change its position, the statolith puts more pressure on one of the four flagella groups than on the other three. This sensation is transmitted to the ectoderm, which is pervaded by eight long "comb rows" (ctenes). | |
| − | + | The ctenes are formed from rows of cilia, which coalesce with one another in their hundreds and form flagella of up to two millimeters long—the longest cilia known. By erecting these discs in sequence, the ctenophore can use them as an oar, which, when the eight ctenes are properly synchronized, allow it to regain its former position. A flagellum group of statocysts is needed for every quadrant and controls two ctenes as a pacemaker. The rhythm is carried automatically and not by [[nerve]] impulses. | |
| − | + | Whether heightened pressure on the flagella groups raises or lowers the stroke frequency depends on the "disposition" or ''geotaxis'' of the ctenophore; if positive, the frequency of increases in pressure is reduced, so that the ctenophore aligns itself with its mouth pointing downwards and swims away from the water's surface. If negative, the frequency increases, the ctenophore points its front end upwards and swims towards the surface. The "disposition" of the ctenophore is determined by sensations handled by the neuron net. | |
| − | + | ===Tentacles=== | |
| − | + | Most species have two opposing retractable tentacles before the mouth opening, which spring from each sheath to catch prey. On the side they often bear a row of fibrous filaments, which unlike [[cnidaria]] do not contain stinging cells, but colloblasts or "lasso cells." | |
| − | == | + | ===Regeneration=== |
| + | Ctenophora are capable of extraordinary regeneration; even if half of the creature is destroyed, often the remaining half can rebuild itself. The same is true of single organs such as the statoliths, which can be regenerated even after being completely lost. The tentacle and colloblasts are regularly fully regenerated. | ||
| − | + | == Reproduction and life cycle== | |
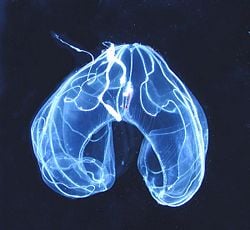
| − | + | [[Image:Juvenile Bolinopsis ctenophore.jpg|thumb|240px|right|Larva of ''Bolenopsis sec.'']] | |
| − | + | Ctenophora reproduce sexually, with the exception of species of the order Platyctenida, which reproduce [[asexual reproduction|asexually]]. Almost all ctenophora are [[hermaphrodite|hermaphroditic]], possessing both male and female reproductive organs, which lie directly under the 'combs' near the small channels of the mesogloea. With almost all species, when triggered by outside lighting conditions the [[gamete]]s are discharged into the surrounding water through small openings in the ectoderm, the ''gonopores'', where [[fertilization]] also takes place. Self-fertilization is somewhat rare and is known only to appear in the genus of ''Mnemiopsis''. A single species, ''Tjalfiella tristoma'', is viviparous; that is, the young grow in a womb. | |
| − | + | After the fertilized eggs have [[cell division|divided]] twice, the ctenophore's later body symmetry has already been set. They develop over a free-floating ''cydippea'' state, which looks very similar between all ctenophora and sometimes is labeled as a [[larva]], although usually in reality it already represents a miniature version of what the creature will grow up to be. Among some extremely specialized groups, such as platyctenides, the ''cydippea'' and adult forms do, however, take separate ecological niches, so that the 'larva' label is more appropriate. | |
| − | == | + | == Prey and predators == |
| − | + | [[Image:Ctenophore2.jpg|thumb|right|300px|A ctenophore looking for food; the mouth is visible on the left]] | |
| − | + | Ctenophora are carnivores that use their tentacles to catch [[plankton]], [[larva]]e, [[worm]]s, [[crustacean]]s, [[cnidaria]], other ctenophora, and sometimes small [[fish]]. The colloblasts or "lasso cells" burst open when prey comes in contact with the tentacle. Sticky threads released from each of the colloblasts will then capture the food. When their tentacles are loaded with food, they can be retracted and wiped off. The food is then carried into the stomach either by mucus or inner cilia. The species of the genus ''Haeckelia'' feed almost exclusively on cnidaria, but do not digest their cnidocytes; instead they build them into their own tentacles as kleptocnides. This 'theft' baffled [[zoology|zoologists]] for a long time as they falsely assumed ctenophora were also capable of forming cnidocytes, and thus their placement with the cnidarians. | |
| − | + | Not all varieties have tentacles. Some instead use their muscular mouth lobes to catch food, which are simply drawn over their prey. | |
| − | + | Like many cnidaria, ctenophora sometimes live with various [[algae]], which supply them with energy-rich [[carbohydrate]]s through [[photosynthesis]] in a [[symbiosis|symbiotic]] relationship. [[Parasitism]] has only been observed in a single species, ''Lampea pancerina'', which lives in [[tunicate]]s. | |
| − | + | Among the species who prey on ctenophora are cnidaria, [[sea turtle]]s, various fish such as [[mackerel]]s and [[lumpfish]], [[seabird]]s, and other ctenophora. | |
| − | + | == Habitat == | |
| − | + | All ctenophora live in the sea, where they are found to depths of up to three kilometers. Their habitat is fixed primarily by the ocean currents, in particular tides. A few species even appear in the North Sea, such as the sea gooseberry (''Pleurobrachia pileus'') or ''Beroe gracilis''. | |
| − | + | The most well-known species live as [[plankton]] in the ocean layers near the surface. However, as they are largely transparent, extremely fragile, and rarely grow longer than a few millimeters, they are unknown to most people. On the coast, the nodula ''Pleurobrachia'' species are found most frequently, of which the sea gooseberries are part. ''Bolinopsis'', ''Mnemiopsis'', and the tentacle-less ''Beroe'' can also be found fairly frequently. | |
| − | + | About 35 species are known to live on the sea bed. These species are ordered in the taxon of [[platyctenida]]e, due to their flattened forms, which more closely resemble slugs or flatworms (Platyhelminthes) than jellyfish. | |
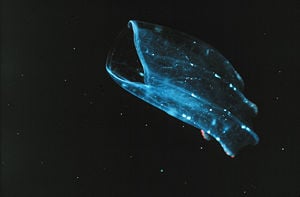
| − | + | The ctenophora, known as ''Mertensia ovum'', make up the most predominant group of plankton in the [[arctic]] waters. | |
| − | |||
| − | == | + | == Ctenophore as an invasive species == |
| − | |||
| − | |||
| − | + | Although ctenophora are generally hardly noticeable and their influence on an [[ecosystem]] is ostensibly very low, they can still do significant damage when they find themselves in non-native waters. The North Atlantic species ''Mneiopsis leidyi'' first appeared in the Black Sea, possibly brought by ships' ballast water, and by 1988 had spread throughout the Black Sea (Shiganova 1998). By the 1990s, the highly productive Black Sea ecosystem was dominated by a "dead end gelatinous food web" (Shiganova 1998). A full ecosystem fisheries collapse had occurred, including the [[anchovy]] fishing industry, as the ctenophore managed to outcompete the native fish for food (Shiganova 1998). It did this largely by eating the zooplankton in the water before the fish eggs had hatched, leaving little for the fry and fingerlings, although even the adult fish were in poor condition as a result of the competition (Mills 2005). The [[biomass]] of (inedible) ctenophora in the Black Sea reached more than a million tons at its highest point of the crisis. | |
| − | + | Through the similarly sudden appearance in 1997 of another ctenophore, ''Beroe ovata'', which feeds on ''Mneipsis leidyi'', the balance has swung the other way, as ''Mneipsis'' populations have come under control and the ecosystem recovered. However, since then the Black Sea has been occupied by both foreign species. The same scenario with the same species has now begun to be played out in the [[Caspian Sea]]. | |
| − | + | == Classification== | |
| − | + | Sailors have observed ctenophora since ancient times. However, the first recorded sighting only came in 1671, made by a ship's doctor. The Swedish taxonomist [[Carl von Linné]] classified them with other 'primitive' [[invertebrate]]s, such as [[sponge|sea sponges]] (Porifera) or cnidaria, as 'zoophytes' ("animal plants"), alluding to the passive, "plant-like" character of the creatures. The French zoologist [[Georges Cuvier]] supported this classification. Only in the nineteenth century were ctenophora recognized as a standalone taxon. | |
| − | '' | + | [[Image:Bathocyroe fosteri.jpg|thumb|right|250px|A ''Bathocyroe fosteri'' arranging the combs on its upper end towards the side view]] |
| − | + | Ctenophores traditionally classified with the cnidaria in the phylum Coelenterata. This is based on anatomical similarities, and was complicated, as noted above, by the fact that nematocysts (cnidocytes) were found in ctenophora, which actually had come from cnidarian prey. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | ''' | + | The initial classification of ctenophores with cnidarians has been disputed. According to [[cladistics]], currently the leading ordering method, ctenophora are more closely related to the reflectively symmetrical [[bilateria]] than cnidaria. The fact that they have two opposing tentacles, breaking their radial symmetry and making them reflectively symmetrical, supports this. They differ from cnidaria in their possession of true muscle tissue and their "combs." Another important sign of ctenophore's relationship with bilateria is the form of their [[spermatozoa]]. These consist in both groups of a single, large acrosome and a ''subacrosomic perforation disc''. Cnidarian spermatozoa, in contrast, possess several acrosomic vesicles. |
| − | + | The term ''coelenterate'' is no longer recognized as including both cnidarians and ctenophores, which are now separate phyla, but the term is sometimes used for cnidarians. | |
| − | |||
| − | |||
| − | |||
| − | |||
| − | In | + | In a 1997 work, Margulis and Schwartz, revising an earlier model by Thomas Cavalier-Smith, placed the Cnidaria and Ctenophora alone under the Radiata branch of the Eumetazoa subregnum. The latter refers to all the animals except the [[Porifera|sponges]], Trichoplax, and the still poorly understood Mesozoa. |
| − | The soft bodies of the ctenophora, which have no hard parts whatsoever, makes | + | The soft bodies of the ctenophora, which have no hard parts whatsoever, makes fossilization generally very improbable, meaning that the [[phylogeny]] of ctenophora fossils is very sparsely documented. The sole fossil records, of ''Archaeocydippida hunsrueckiana'' and ''Paleoctenophora brasseli'', date from the [[Devonian]] Period; enough details remained in the fine-grained schist of Hunsrück to make identification possible. It is disputed whether the species ''Matianoascus octonarius'', known from the Chengjiang Fauna of the lower [[Cambrian]] Period, is a member of the ctenophore phylum, while three species, ''Ctenorhabdotus capulus'', ''Fasciculus vesanus'', and ''Xanioascus canadensis'', are known from the Cambrian Burgess Shale. |
| − | == | + | === Classes and orders of Ctenophora=== |
Currently about a hundred species are known, which are traditionally split into the classes of Tentaculata (also known as Tentaculifera) and Nuda (also known as Atentaculata). | Currently about a hundred species are known, which are traditionally split into the classes of Tentaculata (also known as Tentaculifera) and Nuda (also known as Atentaculata). | ||
| − | *The | + | *The Tentaculata make up by far the largest number of species; as their name implies, they possess tentacles, although these are sometimes vestigial. They are divided into the following six orders: |
| − | ** | + | **Cydippida, which includes the sea gooseberry (''Pleurobrachia pileus'') |
| − | ** | + | **Platyctenida |
| − | ** | + | **Ganeshida |
| − | ** | + | **Thalassocalycida |
| − | ** | + | **Lobata |
| − | ** | + | **Cestida, which includes the Venus' belt (''Cestum veneris'') |
| − | *The | + | *The Nuda class contains only a single order, Beroida, to which the melon jelly (''Beroe gracilis'') belongs. As again the name of the taxon implies, they are distinguished by the complete absence of tentacles. |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
== References == | == References == | ||
| − | *''Much of this article is based on a translation of the [http://de.wikipedia.org/w/index.php?title=Rippenquallen&oldid=15011337 corresponding German-language Wikipedia article] | + | *''Much of this article is based on a translation of the [http://de.wikipedia.org/w/index.php?title=Rippenquallen&oldid=15011337 corresponding German-language Wikipedia article,] retrieved on April 5, 2006.'' |
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| − | |||
| + | * Anderson, D. T. 2001. ''Invertebrate Zoology'', 2nd ed. New York: Oxford Univ. Press. ISBN 0195513681 | ||
| + | * Barnes, R. S. K., P. Calow, P. J. W. Olive, D. W. Golding, J. I. Spicer. 2001. ''The Invertebrates: A Synthesis'', 3rd ed. Blackwell. ISBN 0632047615 | ||
| + | * Brusca, R. C., and G. J. Brusca. 2003. ''Invertebrates'', 2nd ed, Sinauer Associates. ISBN 0878930973 | ||
| + | * Margulis, L., and K. V. Schwartz. 1997, ''Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth''. W. H. Freeman & Company. ISBN 0613923383 | ||
| + | * Martindale, M. Q., and J. Q. Henry. 1997. ''Ctenophora'', in S. F. Gilbert, A. M. Raunio, ''Embryology: Constructing the Organism''. Sinauer Associates. | ||
| + | * Mills, C. 2005. [http://faculty.washington.edu/cemills/Ctenophores.html ''Ctenophores'']. Access date: November 28, 2006. | ||
| + | * Moore, J. 2001. ''An Introduction to the Invertebrates''. Cambridge Univ. Press. ISBN 0521779146 | ||
| + | * Podar, M., S. H. D. Haddock, M. L. Sogin, and G. R. Harbison. 2001. A molecular phylogenetic framework for the phylum Ctenophora using 18S rRNA genes. ''Molecular Phylogenetics and Evolution'' 21: 218. | ||
| + | * Ruppert, E. E., R. S. Fox, and R. P. Barnes. 2004. ''Invertebrate Zoology: A Functional Evolutionary Approach''. Brooks/Cole. ISBN 0030259827 | ||
| + | * Schäfer, W. 1996. ''Ctenophora, Rippenquallen'', in W. Westheide and R. Rieger: ''Spezielle Zoologie Band 1''. Stuttgart: Gustav Fischer Verlag. | ||
| + | * Shiganova, T. A. 1998. [http://faculty.washington.edu/cemills/Shiganova1998.pdf ''Invasion of the Black Sea by the ctenophore ''Mnemiopsis leidyi'' and recent changes in pelagic community structure'']. ''Fisheries Oceanography'' 7 (3/4): 305-310. | ||
| + | * Stanley, G. D., and W. Stürmer. 1983. The first fossil ctenophore from the lower devonian of West Germany. ''Nature'' 303: 518. | ||
| + | * Wenzel, B. 1958. ''Glastiere des Meeres. Rippenquallen (Acnidaria)''. ISBN 3740301899 | ||
| − | {{ | + | {{credit2|Ctenophore|59430669|Coelenterata|89291928}} |
[[Category:Life sciences]] | [[Category:Life sciences]] | ||
| + | [[Category:Animals]] | ||
| + | [[Category:Invertebrates]] | ||
Latest revision as of 13:42, 29 August 2008
| Comb jellies | ||||
|---|---|---|---|---|
 "Ctenophorae" from Ernst Haeckel's Kunstformen der Natur | ||||
| Scientific classification | ||||
| ||||
| Classes | ||||
|
Tentaculata |
Ctenophores (phylum Ctenophora), also known as comb jellies, are marine invertebrates that have eight rows of comb-like cilia on their transparent, gelatinous bodies. They are the largest animal to use cilia for locomotion.
Superficially, ctenophores resemble jellyfish, which belong to the phylum Cnidaria. Indeed, the Ctenophores and the Cnidaria were formerly grouped together as Coelenterata. However, despite their appearance, ctenophores are zoologically not true jellyfish, not least because they lack the characteristic cnidocytes (specialized cells that carry stinging organelles) that characterize the Cnidaria.
The signature characteristic of ctenophores are the comb rows, whereby the closely-spaced cilia in each row, which are fused at the base, are arranged as a stack of combs, called comb plates or ctenes. The word ctenophore (pronounced without the c) comes from Greek, kteno-, kteis, "comb" and -phore, meaning "bearer."
As carnivores, ctenophores are integral in food webs, consuming other ctenophores, small crustaceans, and other marine invertebrates, and being consumed by jellyfish, sea turtles, and other ctenophores, among others. They also provide aesthetic value to humans, whether through the unique light-scattering produced by the rows of cilia, which appear as a changing rainbow of colors moving down the comb rows (Mills 2005), or their gelatinous transparent and sometimes colorful (though generally colorless) bodies, or the bioluminescence of many species. However, invasive species of ctenophores have been known to severely damage ecosystems, including a collapse of the Black Sea fisheries when an accidentally introduced ctenophore species outcompeted the fish for food.
There are more than one hundred varieties of ctenophore spread throughout the worlds' oceans, which form a considerable proportion of the entire plankton biomass. Most centophores are small (a few millimeters to several centimeters in diameter), but a few species have individuals that approach (or exceed) one meter (Mills 2005). Ctenophores live only in marine waters, and can be found from the poles to tropical zones, and from the surface to the deep ocean (Mills 2005). A few species, such as the sea gooseberry (Pleurobrachia pileus), native to the North Sea, have reached such high populations that they clog fishermen's nets, while of other species only a few examples are known. The fragile makeup of ctenophora makes research into their way of life extremely difficult.
Body
Ctenophora are often colorless, except for the coloration caused by algae cells with which they live in symbiosis. This is particularly true of ctenophores that live near the ocean surface.
However, there are species that live in deep waters that can be highly pigmented, such as the Red Tortuga, whose scientific name is not yet established. The Red Tortuga is dark red in color and, like many other ctenophores, can give off light by means of bioluminescence. The coloration may serve as camouflage for the species that live on the sea bed. One species, Eurhamphaea vexilligera, can give off a luminous red tint, which may dissuade predators. Ctenophores that live on the bottom of the ocean are often brightly colored as adults and may look like nudibranches or flatworms; some are colored similarly to their substrate (Mills 2005).
The beating of the eight rows of cilia can also scatter light and cause the appearance of a changing rainbow of colors moving down the comb rows (Mills 2005). This is not to be confused with bioluminescence, whose blue or green light can only be seen in darkness (Mills 2005).
While they often are only a few centimeters long, the species of the Cestum genus can reach an average of one and a half meters.
Ctenophores are considered to be "bi-radially symmetrical", with an underlying bilaterial symmetry, as opposed to the complete radial symmetry of the cnidarians. The main bodily axis running between the mouth and their sensory organ, the statocyst, that lies exactly opposite from the mouth, offers a radial symmetry. This symmetry is superficially broken in the lower part of the creatures by the two tentacles and in the higher part by the digestive chamber, which is separated into several channels. The lower symmetry is shifted round from the higher by ninety degrees, forming a disymmetry or a biradial form.
The body consists of two transparent cell layers, which make up its outer skin (ectoderm) and inner skin (gastroderm). The ectoderm, made up of two cell layers, is mostly covered by a protective layer of slime, excreted by special glands. The gastroderm surrounds a cavity which serves as a stomach and is only accessible by the mouth opening, connected by a long, narrow gullet. Captured quarry is pre-digested in the gullet by strong enzymes and fully decomposed in the stomach. There is no separate exit from the stomach apart from two 'anal pores'—which despite their name, are not used for excretion—so indigestible waste must be expelled via the mouth.
The space between the inner and outer skin is taken up by the mesogloea, a thick, transparent, jelly-like layer made from collagen and connective tissue, pervaded by numerous small channels, which are used for transport and storage of nutrients. The position of the channels varies from species to species, but they mostly run directly underneath the tissues that they serve. The extracellular net of structural protein is kept upright by special cells similar to amoebas.
The mesogloea may also play a role in the lift of the creatures. Flagella found in the channels of the digestive system may serve to pump water in or out of the mesogloea, when osmotic water pressure changes, perhaps because the creature has swum out of saline sea water into coastal brackwater.
Ctenophora do not possess a specific circulatory system, neither do they have any organs for breathing; gas exchange and the excretion of waste products of cell metabolism, such as ammonia, occur over the body's entire surface through simple diffusion. The body is pervaded by a simple net of neurons without a "brain," with the neurons concentrated around the gullet, tentacles, "combs," and statocysts; it is connected with the muscular cells found in the mesogloea and the inner cellular layer of the ectoderm.
Statocyst, comb rows, and their use in movement
Many ctenophora simply let themselves drift with the current. They can, however, also swim short distances by means of the strokes of their flagella and by using their mouth opening as a rudder. They are the largest animals to use their flagella for movement and can reach speeds of about five centimeters per second. A possible adaptive advantage is that constant strokes do not cause vibrations that would alert prey or predators.
Some varieties also use the muscular cells of their mouth lobes to swim, while others move by undulating their body or creeping like flatworms.
The statocyst is a specialized system that serves the ctenophore as a balancing organ and also controls its movement. It can be found on the side of the body turned away from the mouth opening. It is formed by a collection of a few hundred lime cells on one side and four horizontal groups of serpentine flagella, known as the statolith. As outside influences cause the ctenophore to change its position, the statolith puts more pressure on one of the four flagella groups than on the other three. This sensation is transmitted to the ectoderm, which is pervaded by eight long "comb rows" (ctenes).
The ctenes are formed from rows of cilia, which coalesce with one another in their hundreds and form flagella of up to two millimeters long—the longest cilia known. By erecting these discs in sequence, the ctenophore can use them as an oar, which, when the eight ctenes are properly synchronized, allow it to regain its former position. A flagellum group of statocysts is needed for every quadrant and controls two ctenes as a pacemaker. The rhythm is carried automatically and not by nerve impulses.
Whether heightened pressure on the flagella groups raises or lowers the stroke frequency depends on the "disposition" or geotaxis of the ctenophore; if positive, the frequency of increases in pressure is reduced, so that the ctenophore aligns itself with its mouth pointing downwards and swims away from the water's surface. If negative, the frequency increases, the ctenophore points its front end upwards and swims towards the surface. The "disposition" of the ctenophore is determined by sensations handled by the neuron net.
Tentacles
Most species have two opposing retractable tentacles before the mouth opening, which spring from each sheath to catch prey. On the side they often bear a row of fibrous filaments, which unlike cnidaria do not contain stinging cells, but colloblasts or "lasso cells."
Regeneration
Ctenophora are capable of extraordinary regeneration; even if half of the creature is destroyed, often the remaining half can rebuild itself. The same is true of single organs such as the statoliths, which can be regenerated even after being completely lost. The tentacle and colloblasts are regularly fully regenerated.
Reproduction and life cycle
Ctenophora reproduce sexually, with the exception of species of the order Platyctenida, which reproduce asexually. Almost all ctenophora are hermaphroditic, possessing both male and female reproductive organs, which lie directly under the 'combs' near the small channels of the mesogloea. With almost all species, when triggered by outside lighting conditions the gametes are discharged into the surrounding water through small openings in the ectoderm, the gonopores, where fertilization also takes place. Self-fertilization is somewhat rare and is known only to appear in the genus of Mnemiopsis. A single species, Tjalfiella tristoma, is viviparous; that is, the young grow in a womb.
After the fertilized eggs have divided twice, the ctenophore's later body symmetry has already been set. They develop over a free-floating cydippea state, which looks very similar between all ctenophora and sometimes is labeled as a larva, although usually in reality it already represents a miniature version of what the creature will grow up to be. Among some extremely specialized groups, such as platyctenides, the cydippea and adult forms do, however, take separate ecological niches, so that the 'larva' label is more appropriate.
Prey and predators
Ctenophora are carnivores that use their tentacles to catch plankton, larvae, worms, crustaceans, cnidaria, other ctenophora, and sometimes small fish. The colloblasts or "lasso cells" burst open when prey comes in contact with the tentacle. Sticky threads released from each of the colloblasts will then capture the food. When their tentacles are loaded with food, they can be retracted and wiped off. The food is then carried into the stomach either by mucus or inner cilia. The species of the genus Haeckelia feed almost exclusively on cnidaria, but do not digest their cnidocytes; instead they build them into their own tentacles as kleptocnides. This 'theft' baffled zoologists for a long time as they falsely assumed ctenophora were also capable of forming cnidocytes, and thus their placement with the cnidarians.
Not all varieties have tentacles. Some instead use their muscular mouth lobes to catch food, which are simply drawn over their prey.
Like many cnidaria, ctenophora sometimes live with various algae, which supply them with energy-rich carbohydrates through photosynthesis in a symbiotic relationship. Parasitism has only been observed in a single species, Lampea pancerina, which lives in tunicates.
Among the species who prey on ctenophora are cnidaria, sea turtles, various fish such as mackerels and lumpfish, seabirds, and other ctenophora.
Habitat
All ctenophora live in the sea, where they are found to depths of up to three kilometers. Their habitat is fixed primarily by the ocean currents, in particular tides. A few species even appear in the North Sea, such as the sea gooseberry (Pleurobrachia pileus) or Beroe gracilis.
The most well-known species live as plankton in the ocean layers near the surface. However, as they are largely transparent, extremely fragile, and rarely grow longer than a few millimeters, they are unknown to most people. On the coast, the nodula Pleurobrachia species are found most frequently, of which the sea gooseberries are part. Bolinopsis, Mnemiopsis, and the tentacle-less Beroe can also be found fairly frequently.
About 35 species are known to live on the sea bed. These species are ordered in the taxon of platyctenidae, due to their flattened forms, which more closely resemble slugs or flatworms (Platyhelminthes) than jellyfish.
The ctenophora, known as Mertensia ovum, make up the most predominant group of plankton in the arctic waters.
Ctenophore as an invasive species
Although ctenophora are generally hardly noticeable and their influence on an ecosystem is ostensibly very low, they can still do significant damage when they find themselves in non-native waters. The North Atlantic species Mneiopsis leidyi first appeared in the Black Sea, possibly brought by ships' ballast water, and by 1988 had spread throughout the Black Sea (Shiganova 1998). By the 1990s, the highly productive Black Sea ecosystem was dominated by a "dead end gelatinous food web" (Shiganova 1998). A full ecosystem fisheries collapse had occurred, including the anchovy fishing industry, as the ctenophore managed to outcompete the native fish for food (Shiganova 1998). It did this largely by eating the zooplankton in the water before the fish eggs had hatched, leaving little for the fry and fingerlings, although even the adult fish were in poor condition as a result of the competition (Mills 2005). The biomass of (inedible) ctenophora in the Black Sea reached more than a million tons at its highest point of the crisis.
Through the similarly sudden appearance in 1997 of another ctenophore, Beroe ovata, which feeds on Mneipsis leidyi, the balance has swung the other way, as Mneipsis populations have come under control and the ecosystem recovered. However, since then the Black Sea has been occupied by both foreign species. The same scenario with the same species has now begun to be played out in the Caspian Sea.
Classification
Sailors have observed ctenophora since ancient times. However, the first recorded sighting only came in 1671, made by a ship's doctor. The Swedish taxonomist Carl von Linné classified them with other 'primitive' invertebrates, such as sea sponges (Porifera) or cnidaria, as 'zoophytes' ("animal plants"), alluding to the passive, "plant-like" character of the creatures. The French zoologist Georges Cuvier supported this classification. Only in the nineteenth century were ctenophora recognized as a standalone taxon.
Ctenophores traditionally classified with the cnidaria in the phylum Coelenterata. This is based on anatomical similarities, and was complicated, as noted above, by the fact that nematocysts (cnidocytes) were found in ctenophora, which actually had come from cnidarian prey.
The initial classification of ctenophores with cnidarians has been disputed. According to cladistics, currently the leading ordering method, ctenophora are more closely related to the reflectively symmetrical bilateria than cnidaria. The fact that they have two opposing tentacles, breaking their radial symmetry and making them reflectively symmetrical, supports this. They differ from cnidaria in their possession of true muscle tissue and their "combs." Another important sign of ctenophore's relationship with bilateria is the form of their spermatozoa. These consist in both groups of a single, large acrosome and a subacrosomic perforation disc. Cnidarian spermatozoa, in contrast, possess several acrosomic vesicles.
The term coelenterate is no longer recognized as including both cnidarians and ctenophores, which are now separate phyla, but the term is sometimes used for cnidarians.
In a 1997 work, Margulis and Schwartz, revising an earlier model by Thomas Cavalier-Smith, placed the Cnidaria and Ctenophora alone under the Radiata branch of the Eumetazoa subregnum. The latter refers to all the animals except the sponges, Trichoplax, and the still poorly understood Mesozoa.
The soft bodies of the ctenophora, which have no hard parts whatsoever, makes fossilization generally very improbable, meaning that the phylogeny of ctenophora fossils is very sparsely documented. The sole fossil records, of Archaeocydippida hunsrueckiana and Paleoctenophora brasseli, date from the Devonian Period; enough details remained in the fine-grained schist of Hunsrück to make identification possible. It is disputed whether the species Matianoascus octonarius, known from the Chengjiang Fauna of the lower Cambrian Period, is a member of the ctenophore phylum, while three species, Ctenorhabdotus capulus, Fasciculus vesanus, and Xanioascus canadensis, are known from the Cambrian Burgess Shale.
Classes and orders of Ctenophora
Currently about a hundred species are known, which are traditionally split into the classes of Tentaculata (also known as Tentaculifera) and Nuda (also known as Atentaculata).
- The Tentaculata make up by far the largest number of species; as their name implies, they possess tentacles, although these are sometimes vestigial. They are divided into the following six orders:
- Cydippida, which includes the sea gooseberry (Pleurobrachia pileus)
- Platyctenida
- Ganeshida
- Thalassocalycida
- Lobata
- Cestida, which includes the Venus' belt (Cestum veneris)
- The Nuda class contains only a single order, Beroida, to which the melon jelly (Beroe gracilis) belongs. As again the name of the taxon implies, they are distinguished by the complete absence of tentacles.
ReferencesISBN links support NWE through referral fees
- Much of this article is based on a translation of the corresponding German-language Wikipedia article, retrieved on April 5, 2006.
- Anderson, D. T. 2001. Invertebrate Zoology, 2nd ed. New York: Oxford Univ. Press. ISBN 0195513681
- Barnes, R. S. K., P. Calow, P. J. W. Olive, D. W. Golding, J. I. Spicer. 2001. The Invertebrates: A Synthesis, 3rd ed. Blackwell. ISBN 0632047615
- Brusca, R. C., and G. J. Brusca. 2003. Invertebrates, 2nd ed, Sinauer Associates. ISBN 0878930973
- Margulis, L., and K. V. Schwartz. 1997, Five Kingdoms: An Illustrated Guide to the Phyla of Life on Earth. W. H. Freeman & Company. ISBN 0613923383
- Martindale, M. Q., and J. Q. Henry. 1997. Ctenophora, in S. F. Gilbert, A. M. Raunio, Embryology: Constructing the Organism. Sinauer Associates.
- Mills, C. 2005. Ctenophores. Access date: November 28, 2006.
- Moore, J. 2001. An Introduction to the Invertebrates. Cambridge Univ. Press. ISBN 0521779146
- Podar, M., S. H. D. Haddock, M. L. Sogin, and G. R. Harbison. 2001. A molecular phylogenetic framework for the phylum Ctenophora using 18S rRNA genes. Molecular Phylogenetics and Evolution 21: 218.
- Ruppert, E. E., R. S. Fox, and R. P. Barnes. 2004. Invertebrate Zoology: A Functional Evolutionary Approach. Brooks/Cole. ISBN 0030259827
- Schäfer, W. 1996. Ctenophora, Rippenquallen, in W. Westheide and R. Rieger: Spezielle Zoologie Band 1. Stuttgart: Gustav Fischer Verlag.
- Shiganova, T. A. 1998. Invasion of the Black Sea by the ctenophore Mnemiopsis leidyi and recent changes in pelagic community structure. Fisheries Oceanography 7 (3/4): 305-310.
- Stanley, G. D., and W. Stürmer. 1983. The first fossil ctenophore from the lower devonian of West Germany. Nature 303: 518.
- Wenzel, B. 1958. Glastiere des Meeres. Rippenquallen (Acnidaria). ISBN 3740301899
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.