Crystallization
Crystallization is the (natural or artificial) process of formation of solid crystals from a homogeneous solution or melt, or more rarely directly from a gas. This process is often used as a technique to separate a solute from a liquid solution, bringing it into a pure crystalline phase.
Process
For a solute to crystallize out of a solution, the solution must be supersaturated with the solute. This means that the solution has to contain more solute entities (atoms, molecules, or ions) dissolved than it would contain under the equilibrium conditions (of a saturated solution).
The crystallization process consists of two major steps: nucleation and crystal growth. In the nucleation step, the solute molecules dispersed in the solvent start to gather into clusters (on the nanometer scale). When these clusters become stable, they constitute the nuclei. However, when the clusters are not stable, they redissolve. Therefore, the clusters need to reach a critical size to become stable nuclei. The critical size is dictated by the prevailing conditions, such as temperature and supersaturation. It is at the stage of nucleation that the atoms or molecules arrange themselves in a particular periodic manner that defines the crystal structure.[1]
Crystal growth corresponds to growth of the nuclei that succeed in achieving critical cluster size. Nucleation and growth continue to occur simultaneously as long as the solution is supersaturated with the solute.
Supersaturation is the driving force of the crystallization process—the rates of nucleation and growth are driven by supersaturation within the solution. Depending upon the conditions, either nucleation or growth may predominate over the other, and as a result, crystals with different sizes and shapes are obtained. (The control of crystal size and shape constitutes one of the main challenges in industrial manufacturing, such as for pharmaceuticals.) Once the solution is no longer supersaturated, the solid-liquid system reaches equilibrium and crystallization is complete, unless the operating conditions are modified from equilibrium so that the solution becomes supersaturated again.
Many compounds can crystallize with different crystal structures, a phenomenon called polymorphism. Each crystal polymorph is a different thermodynamic solid state. Crystal polymorphs of the same compound exhibit different physical properties, such as dissolution rate, shape (angles between facets and facet growth rates), melting point, and so on. For this reason, polymorphism is of major importance in the industrial manufacture of crystalline products.
Crystallization in nature
There are many examples of crystallization in nature, some of which are noted below.
Examples of crystallization on the geological time scale:
- Formation of minerals, including gemstones.
- Formation of stalactites and stalagmites.
Examples of crystallization on ordinary time scales:
- Formation of snowflakes.
- Crystallization of honey.
Artificial methods
For artificial crystallization of a solute from solution, the conditions must be adjusted such that the solution becomes supersaturated with the solute. This can be achieved by various methods, such as:
- cooling the solution;
- evaporating part of the solvent;
- adding a second solvent that reduces the solubility of the solute (technique known as anti-solvent or drown-out);
- changing the pH of the solution; and
- performing a chemical reaction.
Applications
Artificial crystallization includes two major groups of applications: crystal production and purification.
Crystal production
From the perspective of the materials industry:
- To meet the demand for crystals that simulate natural crystals, there are methods that accelerate the rate of production and crystal perfection:
- Tiny size crystals:
- Powder, sand and smaller sizes: using methods for powder and controlled (nanotechnology fruits) forms.
- Mass-production: on chemical industry, like salt-powder production.
- Sample production: small production of tiny crystals for material characterization. Controlled recrystallization is an important method to supply unusual crystals, that are needed to reveal the molecular structure and nuclear forces inside a typical molecule of a crystal. Many techniques, like X-ray crystallography and NMR spectroscopy, are widely used in chemistry and biochemistry to determine the structures of an immense variety of molecules, including inorganic compounds and bio-macromolecules.
- Thin film production.
- Powder, sand and smaller sizes: using methods for powder and controlled (nanotechnology fruits) forms.
Examples of mass production of crystalline materials:
- "Powder salt for food" industry;
- Silicon crystal wafer production.
- Production of sucrose from sugar beet, where the sucrose is crystallized out from aqueous solution.
Purification
Well-formed crystals are expected to be pure because each molecule or ion must fit perfectly into the lattice as it leaves the solution. Impurities would normally not fit as well in the lattice, and thus remain in solution preferentially. Hence, molecular recognition is the principle of purification in crystallization. However, there are instances when impurities incorporate into the lattice, thus decreasing the level of purity of the final crystalline product. Also, in some cases, the solvent may be incorporated into the lattice, forming a solvate. In addition, the solvent may be 'trapped' (in liquid state) within the crystal, forming what are known as inclusions.
Thermodynamic view
The nature of a crystallization process is governed by both thermodynamic and kinetic factors, which can make it highly variable and difficult to control. Factors such as impurity level, mixing regime, vessel design, and cooling profile can have a major impact on the size, number, and shape of crystals produced.
Now put yourself in the place of a molecule within a pure and perfect crystal, being heated by an external source. At some sharply defined temperature, a bell rings, you must leave your neighbours, and the complicated architecture of the crystal collapses to that of a liquid. Textbook thermodynamics says that melting occurs because the entropy, S, gain in your system by spatial randomization of the molecules has overcome the enthalpy, H, loss due to breaking the crystal packing forces:
This rule suffers no exceptions when the temperature is rising. By the same token, on cooling the melt, at the very same temperature the bell should ring again, and molecules should click back into the very same crystalline form. The entropy decrease due to the ordering of molecules within the system is overcompensated by the thermal randomization of the surroundings, due to the release of the heat of fusion; the entropy of the universe increases.
But liquids that behave in this way on cooling are the exception rather than the rule; in spite of the second principle of thermodynamics, crystallization usually occurs at lower temperatures (supercooling). This can only mean that a crystal is more easily destroyed than it is formed. Similarly, it is usually much easier to dissolve a perfect crystal in a solvent than to grow again a good crystal from the resulting solution. The nucleation and growth of a crystal are under kinetic, rather than thermodynamic, control.
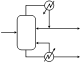
Equipment used for industrial production
1. Tank crystallizer: A hot, saturated solution is placed in an open tank and allowed to cool. Once an adequate level of crystallization is reached, the mother liquor is drained away and the crystals are removed.
Nucleation and size of crystals are difficult to control. Typically, labor costs are very high.
2. Scraped surface crystallizer: One type of scraped surface crystallizer is the Swenson-Walker crystallizer, which consists of an open trough 0.6m wide with a semicircular bottom having a cooling jacket outside. A slow-speed spiral agitator rotates and suspends the growing crystals on turning. The blades pass close to the wall and break off any deposits of crystals on the cooled wall. The product generally has a somewhat wide crystal-size distribution.
3. Double-pipe scraped surface crystallizer: Also called a votator, this type of crystallizer is used in crystallizing ice cream and plasticizing margarine. Cooling water passes in the annular space. An internal agitator is fitted with spring-loaded scrapers that wipe the wall and provide good heat-transfer coefficients.
4. Circulating-liquid evaporator-crystallizer. Also called Oslo crystallizer. Here supersaturation is reached by evaporation. The circulating liquid is drawn by the screw pump down inside the tube side of the condensing stream heater. The heated liquid then flows into the vapor space, where flash evaporation occurs, giving some supersaturation.The vapor leaving is condensed. The supersaturated liquid flows down the downflow tube and then up through the bed of fluidized and agitated crystals, which are growing in size. The leaving saturated liquid then goes back as a recycle stream to the heater, where it is joined by the entering fluid. The larger crystals settle out and slurry of crystals and mother liquid is withdrawn as a product.
5. Circulating-magma vacuum crystallizer. The magma or suspension of crystals is circulated out of the main body through a circulating pipe by a screw pump. The magma flows though a heater, where its temperature is raised 2-6 K. The heated liquor then mixes with body slurry and boiling occurs at the liquid surface. This causes supersaturation in the swirling liquid near the surface, which deposits in the swirling suspended crystals until they leave again via the circulating pipe. The vapors leave through the top. A steam-jet ejector provides vacuum.
Gallery
- Solvent recrystallisation
- X-ray crystals
See also
- Crystal
- Crystal structure
- Crystallite
- Gemstone
- Mineral
- Solute
- Solution
- Solvent
- X-ray crystallography
Notes
- ↑ Note that "crystal structure" is a special term that refers to the relative arrangement of the atoms or molecules, not the macroscopic properties (size and shape) of the crystal, although the latter properties are a result of the internal crystal structure.
ReferencesISBN links support NWE through referral fees
- Geankoplis, C.J. 2003. Transport Processes and Separation Process Principles. 4th ed. Prentice-Hall Inc. ISBN 978-0131013674.
- Glynn, P.D., and E.J. Reardon. 1990. "Solid-solution aqueous-solution equilibria: thermodynamic theory and representation." Amer. J. Sci. 290:164-201.
- Jones, A. G. 2002. Crystallization Process Systems. Oxford: Butterworth-Heinemann. ISBN 978-0750655200.
- Mullin, J. W. 2001. Crystallization. 4th ed. Oxford: Butterworth-Heinemann. ISBN 978-0750648332.
- Myerson, Allan S. 2002. Handbook of Industrial Crystallization. 2nd ed. Boston: Butterworth-Heinemann. ISBN 978-0750670128.
- Stanley, S.J. 2006. "Tomographic imaging during reactive precipitation: mixing with chemical reaction." Chemical Engineering Science 61 (23): 7850-7863.
External links
- Crystallization. Cheresources.com. Retrieved May 13, 2008.
| |||||||||||||
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.