Crystallite

A crystallite is a domain of solid-state matter that has the same structure as a single crystal. Crystallites can vary in size from a few nanometers to several millimeters.
Most solid, crystalline materials that are large enough to see and handle are polycrystalline‚ÄĒthat is, they are made of a large number of single crystals, or crystallites, held together by thin layers of amorphous solid. In addition, the term crystallites is used when referring to tiny crystals observed in glassy volcanic rocks.
Some large single crystals (which are not called crystallites) have been found in nature and others have been produced artificially. They include gems, silicon single crystals for the electronics industry, and single crystals of a nickel-based superalloy for turbojet engines.
Metallurgists often refer to crystallites as grains, and the boundary between crystallites is called the grain boundary. The term "crystallite boundary" is used only rarely. It should be noted that a "powder grain" can be composed of several crystallites.
The strengthening of grain boundaries (or "Hall-Petch strengthening") is a method of strengthening materials by changing their average crystallite size. In other words, by changing grain size, one can strengthen the material. Heat treatment after plastic deformation and changing the rate of solidification are ways to alter grain size.
Natural formations
Coarse-grained rocks are formed very slowly, while fine-grained rocks are formed relatively quickly, on geological time scales. If a rock forms very quickly, such as the solidification of lava ejected from a volcano, there may be no crystals at all. This is how obsidian is formed.
Properties
If the individual crystallites in an object are oriented randomly (that is, if they lack texture), a large enough volume of polycrystalline material will be approximately isotropic. (When referring to the mechanical properties of materials, "isotropic" means having identical values of a property in all crystallographic directions.) In such cases, the simplifying assumptions of continuum mechanics can be applied to real-world solids. However, most manufactured crystalline materials have some alignment of their crystallites, which must be taken into account for accurate predictions of their behavior and characteristics.
A crystalline material can undergo two types of fracture: Intergranular (fracture between grains) or transgranular (fracture through the grains).
As noted above, a powder grain can be made of several crystallites. Thus, the (powder) "grain size" found by laser granulometry can be different from the "grain size" (or, rather, crystallite size) found by X-ray diffraction (for example, Scherrer method), by optical microscopy under polarized light, or by scanning electron microscopy(backscattered electrons).
Generally, polycrystals cannot be superheated; they will melt promptly once they are brought to a high enough temperature. This is because grain boundaries are amorphous and serve as nucleation points for the liquid phase. By contrast, if no solid nucleus is present as a liquid cools, it tends to become supercooled. Since this is undesirable for mechanical materials, alloy designers often take steps against it.
Grain boundaries
Grain boundaries are interfaces where crystals of different orientations meet. A grain boundary is a single-phase interface, with crystals on each side of the boundary being identical except in orientation. Grain boundary areas contain atoms that have been perturbed from their original lattice sites, dislocations, and impurities that have migrated to the lower energy grain boundary. Also, because grain boundaries are defects in the crystal structure, they tend to decrease the electrical and thermal conductivity of the material.
Grain boundaries are generally only a few nanometers wide. In common materials, crystallites are large enough that grain boundaries account for a small fraction of the material. However, very small grain sizes are achievable. In nanocrystalline solids, grain boundaries become a significant volume fraction of the material, with profound effects on such properties as diffusion and plasticity. In the limit of small crystallites, as the volume fraction of grain boundaries approaches 100 percent, the material ceases to have crystalline character and becomes an amorphous solid.
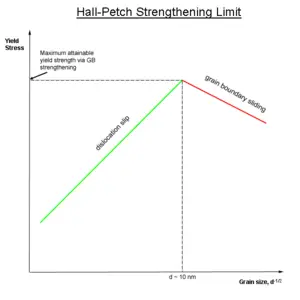
Grain boundaries disrupt the motion of dislocations through a polycrystalline material, and the number of dislocations within a grain have an effect on how easily the dislocations can traverse grain boundaries and travel from grain to grain. Based on this knowledge, the strength of a material can be improved by reducing crystallite size. It can often be achieved without sacrificing toughness of the material, because the smaller grains create more obstacles per unit area of slip plane. This relationship between crystallite size and strength of the material is given by the Hall-Petch relationship. Methods of altering grain size and strengthening grain boundaries include heat treatment after plastic deformation and changing the rate of solidification.[1]
It should be noted that there is a limit to strengthening of a material by reducing grain size. Experiments have shown that the microstructure with the highest yield strength has a grain size of about 10 nanometers. Grains smaller than this size undergo another yielding mechanism, grain boundary sliding. Nonetheless, producing materials with this ideal grain size is difficult because only thin films can be reliably produced with grains of this size.
The high interfacial energy and relatively weak bonding in most grain boundaries make them preferred sites for the onset of corrosion and for the precipitation of new phases from the solid.
Grain boundary migration plays an important role in many of the mechanisms of creep. Grain boundary migration occurs when a shear stress acts on the grain boundary plane and causes the grains to slide. This means that fine-grained materials actually have a poor resistance to creep compared to coarser grains, especially at high temperatures, because smaller grains contain more atoms in grain boundary sites. Grain boundaries also cause deformation in that they are sources and sinks of point defects. Voids in a material tend to gather in a grain boundary, and if this happens to a critical extent, the material could fracture.
Grain boundaries are also present in magnetic domains in magnetic materials. A computer hard disk, for example, is made of a hard ferromagnetic material that contains regions of atoms whose magnetic moments can be realigned by an inductive head. The magnetization varies from region to region, and the misalignment between these regions forms boundaries that are key to data storage. The inductive head measures the orientation of the magnetic moments of these domain regions and reads out either a ‚Äú1‚ÄĚ or ‚Äú0.‚ÄĚ These bits are the data being read. Grain size is important in this technology because it limits the number of bits that can fit on one hard disk. The smaller the grain sizes, the more data that can be stored.
Because of the dangers of grain boundaries in certain materials such as superalloy turbine blades, great technological leaps were made to minimize as much as possible the effect of grain boundaries in the blades. The result was directional solidification processing in which grain boundaries were eliminated by producing columnar grain structures aligned parallel to the axis of the blade, since this is usually the direction of maximum tensile stress felt by a blade during its rotation in an airplane. The resulting turbine blades consisted of a single grain, improving reliability.
If a grain boundary is considered geometrically as an interface of a single crystal cut into two parts, one of which is rotated, five variables are required to define the grain boundary. The first two numbers come from the unit vector that specifies a rotation axis. The third number designates the angle of rotation of the grain. The final two numbers specify the plane of the grain boundary (or a unit vector that is normal to this plane).
Grain refinement
Grain refinement is the set of techniques used in metallurgy to strengthen grain boundaries. The specific techniques and corresponding mechanisms vary based on the materials being considered.
One method for controlling grain size in aluminum alloys is by introducing particles to serve as nucleants, such as aluminum-titanium (with 5 percent titanium). Grains will grow via heterogeneous nucleation; that is, for a given degree of undercooling beneath the melting temperature, aluminum particles in the melt will nucleate on the surface of the added particles. Grains will grow in the form of dendrites growing radially away from the surface of the nucleant. Solute particles can then be added (called grain refiners) which limit the growth of dendrites, leading to grain refinement.[2]
See also
Notes
- ‚ÜĎ William D. Callister, Fundamentals of Materials Science and Engineering: An Integrated Approach, 2nd ed. (Hoboken, NJ: John Wiley & Sons, 2005, ISBN 9780471470144).
- ‚ÜĎ K.T. Kashyap and T. Chandrashekar, Effects and mechanisms of grain refinement in aluminum alloys, Bulletin of Materials Science, vol 24.
ReferencesISBN links support NWE through referral fees
- Allen, Samuel M., and Edwin L. Thomas. The Structure of Materials. MIT Series in Materials Science and Engineering. New York: John Wiley, 1998. ISBN 0471000825
- Callister, William D. Fundamentals of Materials Science and Engineering: An Integrated Approach, 2nd ed. Hoboken, NJ: John Wiley & Sons, 2005. ISBN 978-0471470144
- Jiles, David. Introduction to Magnetism and Magnetic Materials. London: Chapman and Hall, 1991. ISBN 0412386402
- Pellant, Chris. Rocks and Minerals. Smithsonian Handbooks. New York: Dorling Kindersley, 2002. ISBN 0789491060
- Rohrer, Gregory S. Structure and Bonding in Crystalline Materials. Cambridge, UK: Cambridge University Press, 2001. ISBN 0521663792
Credits
New World Encyclopedia writers and editors rewrote and completed the Wikipedia article in accordance with New World Encyclopedia standards. This article abides by terms of the Creative Commons CC-by-sa 3.0 License (CC-by-sa), which may be used and disseminated with proper attribution. Credit is due under the terms of this license that can reference both the New World Encyclopedia contributors and the selfless volunteer contributors of the Wikimedia Foundation. To cite this article click here for a list of acceptable citing formats.The history of earlier contributions by wikipedians is accessible to researchers here:
The history of this article since it was imported to New World Encyclopedia:
Note: Some restrictions may apply to use of individual images which are separately licensed.